��Ŀ����
����Ŀ����ѧ��һֱ���������˹��̵������о��������ж��ַ�����
������һ��1918�꣬�¹���ѧ�ҹ���������ҵ�ϳɰ���N2(g)+3H2(g)![]() 2NH3(g) H<0���ķ������ٻ�ŵ������ѧ����
2NH3(g) H<0���ķ������ٻ�ŵ������ѧ����
��1������1molN2��3molH2����1L���ܱ������У�5min��N2��Ũ��Ϊ0.8mol/L�����ʱ������N2��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ_____mol/(L��min)��
��2����һ���¶��µĶ����ܱ������з���������Ӧ������������˵����Ӧ�Ѿ��ﵽƽ��״̬����____��
a. v(N2)��=3v(H2)��
b. ������������ܶȲ���ʱ����仯
c. ����������ķ�����������ʱ����仯
d. �����������ƽ����Է�����������ʱ����仯
��3���ϳɰ���Ӧ����������ѡ���У�������������ԭ�����͵���________��
��ʹ�ô��� �ڸ��� �۸�ѹ �ܼ�ʱ������Һ������ϵ�з������
A. �٢� B. �ڢ� C. �ۢ� D. �ڢ�
����������1998�꣬��λϣ����ѧ������˵��ϳɰ�����˼·��
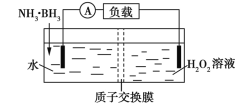
��4�����ø����ӵ����Ե�SCY�մ�(�ܴ���H+)Ϊ���ʣ�ʵ���˸���(570��)��ѹ�¸�ת���ʵĵ�ⷨ�ϳɰ���ת���ʿɴﵽ78����װ������ͼ��
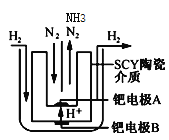
�ٵ缫A�ǵ��ص�___��(����������������)��������ӦʽΪ________��
�������������µ����˹��̵����о��������ڳ��¡���ѹ�����������£�N2�ڴ���������ˮ������Ӧ��ֱ�����ɰ�����������
��֪��N2(g)+3H2(g) ![]() 2NH3(g) ��H=��92 kJ��mol
2NH3(g) ��H=��92 kJ��mol
2H2(g)+O2(g)=2H2O(1) ��H=��571.6 kJ��mol
��5��д�������̵���Ӧ���Ȼ�ѧ����ʽ___________��
���𰸡�0.04 c d C �� H2��2e��=2H+ 2N2(g)+6H2O(I)=4NH3(g)+3O2(g) ��H=+1530.8kJ/mol
��������
��1������![]() ������N2��Ũ�ȱ仯��ʾ�ķ�Ӧ���ʣ�
������N2��Ũ�ȱ仯��ʾ�ķ�Ӧ���ʣ�
��2������ƽ��״̬��־�жϣ�
��3����������ԭ���ʺϽ���ƽ���ƶ���
��4��ԭ����е����������ϵõ����ӷ�����ԭ��Ӧ�������ڸ�����ʧ���ӷ���������Ӧ��
��5�����ݸ�˹���ɼ���2N2(g)+6H2O(I)=4NH3(g)+3O2(g)���ʱ䣻
��1������1molN2��3molH2����1L���ܱ������У�5min��N2��Ũ��Ϊ0.8mol/L��![]() 0.04 mol/(L��min)
0.04 mol/(L��min)
��2��a. ���淴Ӧ����֮�ȵ���ϵ���ȣ���Ӧ�ﵽƽ�⣬����3v(N2)��=v(H2)���ﵽƽ�⣬�ʲ�ѡa��
b. N2(g)+3H2(g)![]() 2NH3(g)�ں��������н��У��ܶ��Ǻ�����������������ܶȲ���ʱ����仯����һ��ƽ�⣬�ʲ�ѡb��
2NH3(g)�ں��������н��У��ܶ��Ǻ�����������������ܶȲ���ʱ����仯����һ��ƽ�⣬�ʲ�ѡb��
c. N2(g)+3H2(g)![]() 2NH3(g)��Ӧ����������������DZ�����������������ķ�����������ʱ����仯��һ��ƽ�⣬��ѡc��
2NH3(g)��Ӧ����������������DZ�����������������ķ�����������ʱ����仯��һ��ƽ�⣬��ѡc��
d. ����![]() �������������ƽ����Է��������DZ������������������ƽ����Է�����������ʱ����仯��һ��ƽ�⣬��ѡd��
�������������ƽ����Է��������DZ������������������ƽ����Է�����������ʱ����仯��һ��ƽ�⣬��ѡd��
��3���ٴ�������ʹƽ���ƶ�����������������ԭ�����ͣ��ʲ�ѡ�٣� ��N2(g)+3H2(g)![]() 2NH3(g) H<0�������¶ȣ�ƽ�������ƶ��������ڰ��������ɣ���������������ԭ�����ͣ��ʲ�ѡ�ڣ� ��N2(g)+3H2(g)
2NH3(g) H<0�������¶ȣ�ƽ�������ƶ��������ڰ��������ɣ���������������ԭ�����ͣ��ʲ�ѡ�ڣ� ��N2(g)+3H2(g)![]() 2NH3(g)������ѹǿ��ƽ�������ƶ������ڰ��������ɣ�������������ԭ�����ͣ���ѡ�ۣ��ܼ�ʱ������Һ������ϵ�з��������ƽ�������ƶ������ڰ��������ɣ�������������ԭ�����ͣ���ѡ�ܣ���C��ȷ��
2NH3(g)������ѹǿ��ƽ�������ƶ������ڰ��������ɣ�������������ԭ�����ͣ���ѡ�ۣ��ܼ�ʱ������Һ������ϵ�з��������ƽ�������ƶ������ڰ��������ɣ�������������ԭ�����ͣ���ѡ�ܣ���C��ȷ��
��4������ͼʾ��B�缫������ʧ�������������ӣ�����B��������A�������������缫��Ӧʽ��H2��2e��=2H+��
��5����N2(g)+3H2(g) ![]() 2NH3(g) ��H=��92 kJ��mol
2NH3(g) ��H=��92 kJ��mol
��2H2(g)+O2(g)=2H2O(1) ��H=��571.6 kJ��mol
���ݸ�˹���ɢ١�2���ڡ�3�� 2N2(g)+6H2O(I)=4NH3(g)+3O2(g) ��H=+1530.8kJ/mol

 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д�����Ŀ����ͨ����н�������ʵ�飺
ʵ�� ���� |
|
|
|
|
���� | Fe����������� ��ɫ���壬��Һ�� �Ϸ������ɫ | Fe�������� �Ա仯 | ����������ɫ���ݣ����Ⱥ�Cu���������ɫ���壬��Һ���Ϸ������ɫ | Cu��������� ��ɫ���� |
��ش��������⣺
��1��������������ɫ��Ϊ����ɫ������ɫ������__________________�������ʽ����
��2�����е�����˵��Fe������______��������ԭ����________________________��
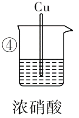
��3���ԱȢ٢��е�����______����������������������˵��ϡ�����������ǿ��Ũ���ᡣ
��4���ԱȢۢ��е�����˵�������ԣ�ϡ����______������>������<����Ũ���ᡣ
��5�������ڼ���ʱ�Ļ�ѧ��Ӧ����ʽΪ________________________���˷�Ӧ��ϡ��������ֳ��������⣬�����ֳ�____________��