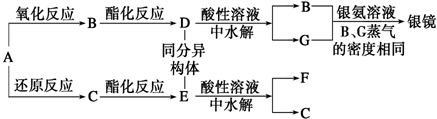
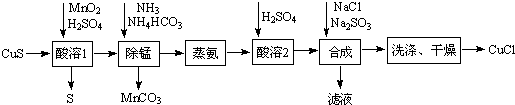
��Ŀ����
8��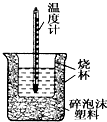 50mL0.05mol•L-1������50mL0.55mol•L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL0.05mol•L-1������50mL0.55mol•L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺��1����ʵ��װ���Ͽ���ͼ����ȱ��һ�ֲ��������ǻ��β����������
��2���ձ�����������ĭ���ϵ������Ǽ���ʵ������е�������ʧ��
��3�����ձ����粻��Ӳֽ�壬��õ��к�����ֵƫС���ƫ��ƫС������Ӱ�족����
��4��ʵ���и���60mL 0.50mol•L-1�����50mL 0.55mol•L-1 NaOH��Һ���з�Ӧ��������ʵ����ȣ�
���ų�����������ȣ����ȡ�����ȡ����������к�����ȣ����ȡ�����ȡ�����
��5������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к�����ֵ��ƫС����50mL 0.50mol•L-1 NaOH��Һ��������ʵ�飬��õ��к�����ֵ����Ӱ�죮���ƫ����ƫС������Ӱ�족��
���� ��1���������ȼƵĹ������жϸ�װ�õ�ȱ��������
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3������Ӳֽ�壬����һ��������ɢʧ��
��4����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��5��������ʵ��������������к�����ָǿ���ǿ�Ӧ����1molˮʱ�ų��������������������أ�
��� �⣺��1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β����������
�ʴ�Ϊ�����β����������
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮����������ĭ���ϵ������Ǽ���ʵ������е�������ʧ��
�ʴ�Ϊ������ʵ������е�������ʧ��
��3�����ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С��
�ʴ�Ϊ��ƫС��
��4����Ӧ�ų����������������Լ�������Ķ����йأ���60mL 0.50mol•L-1�����50mL 0.55mol•L-1 NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ��к�����ȣ�
�ʴ�Ϊ������ȣ���ȣ�
��5��һˮ�ϰ�Ϊ����������Ϊ���ȹ��̣������ð�ˮ����ϡ����������Һ��Ӧ����Ӧ�ų�������ƫС����õ��к�����ֵ��ƫС���к�����ָǿ���ǿ�Ӧ����1molˮʱ�ų��������������������أ���������50mL 0.50mol•L-1NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��Ӱ�죻
�ʴ𰸣�ƫС����Ӱ�죮
���� ���⿼��ѧ���й��к��ȵIJⶨ֪ʶ��ע���к������ᡢ������ʵ����أ��ѶȲ���

��CH4��g��+$\frac{1}{2}$O2��g���TCO��g��+2H2��g������H1=-36kJ•mol-1
��CH4��g��+H2O��g���TCO��g��+3H2��g������H2=+216kJ•mol-1
��1����Ӧ���вμӷ�Ӧ��CH4��g����H2O��g����������С�ڣ�����ڡ�����С�ڡ����ڡ������ɵ�CO��g����H2��g������������
��2����������ˮ�����Ļ�����������������ʵ�������Ϊx������д���пհף�
| ��� | x��O2�� | ��O2��Ӧ�� ��Q1/kJ�� | ��H2O��Ӧ�� ��Q2/kJ�� | �ܷ�Ӧ�� ��Q3/kJ�� |
| I | 0.2 | -18 | ||
| �� | -36 | +72 |
��O-H��O ��N-H��N ��F-H��F ��O-H��N��
| A�� | �ۣ��٣��ܣ��� | B�� | �٣��ڣ��ۣ��� | C�� | �ۣ��ڣ��٣��� | D�� | �٣��ܣ��ۣ��� |

 ��
��