��Ŀ����
ij�Ͻ�(����ͭ����)��ͭ���������ʵ���֮��Ϊy mol������Cu�����ʵ�������Ϊa������ȫ��Ͷ��50 mL b mol��L��1��������Һ�У�����ʹ���ַ�Ӧ(����NO��Ψһ�Ļ�ԭ����)������˵����ȷ����
| A����������ʣ�࣬����Һ���ٵ�������������ܽ� |
| B��������ȫ���ܽ⣬����Һ��һ������Fe3�� |
| C��������ȫ���ܽ⣬�Ҳ���336 mL����(��״��)����b = 0.3 |
| D������Һ�н�������ֻ��Fe3����Cu2��ʱ����a��b�Ĺ�ϵΪ��b��80y(1��a/3) |
D
A����ȷ����Һ�к���NO3�����������ᣬ���Լ�������������B��һ����ȷ��Ҳ�������������ӡ�336mlNO��0.015mol������0.05b��0.015mol��C����ȷ���Ͻ�������ͭ�����ʵ����ֱ���ymol��aymol��aymol��ʧȥ������3ymol��3aymol��2aymol��3ymol��aymol����ԭ��������ymol��ay/3mol������һ����0.05b��y��ay/3��3y��3ay��2ay�����b��80 y (1��a/3)����ѡD��

��ϰ��ϵ�д�
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�
�����Ŀ
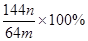 ����ѧ���ļ����Ƿ���ȷ�� ����˵������ ��
����ѧ���ļ����Ƿ���ȷ�� ����˵������ ��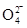 )��
)��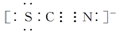
 Fe2(SO4)3 + 3SO2��+ 6H2O���������̽�����ش�������⡣
Fe2(SO4)3 + 3SO2��+ 6H2O���������̽�����ش�������⡣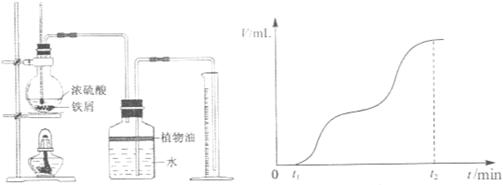
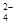 ��ij��Һ��(ֻ��Դ��ˮ��������Ӳ�����)��������п��,����ʹ���ַ�Ӧ,����˵������ȷ���ǣ� ��
��ij��Һ��(ֻ��Դ��ˮ��������Ӳ�����)��������п��,����ʹ���ַ�Ӧ,����˵������ȷ���ǣ� ��
