��Ŀ����
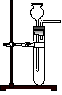
��������װ��̽�����������ʡ�
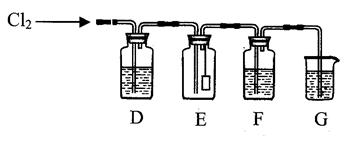
��1��д��ʵ������ȡC12�Ļ�ѧ����ʽ ��
ʵ������ȡ������Ҫ�õ��������� ����д��ţ���
�ٷ�Һ©���ڼ�ʽ�ζ��ܢ�ƾ��Ƣ�Բ����ƿ�ݳ���©��
��2����E�еĸ�����ɫ����������ɫ����D��ʢ�е��Լ��� ��
��3��G��ʢ��NaOH��Һ��д��������Ӧ�����ӷ���ʽ ��
��4��F��ʢ������ˮ��C12ͨ����ɹ۲쵽��Һ����ɫ��Ϊ ɫ��˵����Һ�д��� ��������Һ���뺬�з�̪������������Һ�У���Һ��ɫ��ȥ�����ʵ��̽����ɫ��ȥ��ԭ��
��
��5����F��ʢ�е����廯������Һ��ͨ�����C12��д�����ܷ�����Ӧ������ ����ʽ ��
����ʽ ��

��1��д��ʵ������ȡC12�Ļ�ѧ����ʽ ��
ʵ������ȡ������Ҫ�õ��������� ����д��ţ���
�ٷ�Һ©���ڼ�ʽ�ζ��ܢ�ƾ��Ƣ�Բ����ƿ�ݳ���©��
��2����E�еĸ�����ɫ����������ɫ����D��ʢ�е��Լ��� ��
��3��G��ʢ��NaOH��Һ��д��������Ӧ�����ӷ���ʽ ��
��4��F��ʢ������ˮ��C12ͨ����ɹ۲쵽��Һ����ɫ��Ϊ ɫ��˵����Һ�д��� ��������Һ���뺬�з�̪������������Һ�У���Һ��ɫ��ȥ�����ʵ��̽����ɫ��ȥ��ԭ��
��
��5����F��ʢ�е����廯������Һ��ͨ�����C12��д�����ܷ�����Ӧ������
 ����ʽ ��
����ʽ ��
��

��ϰ��ϵ�д�
�����Ŀ

 �������γ������ݣ��������ɵ���������������Χ������ֹ���ij����ۼ����ţ��� �� ��
�������γ������ݣ��������ɵ���������������Χ������ֹ���ij����ۼ����ţ��� �� ��
 �ζ������յ�ʱ����KMnO4��Һ���29.80mL��
�ζ������յ�ʱ����KMnO4��Һ���29.80mL��
 �����鰱���Ƿ��ռ����ķ����ǣ�������������������ͽ�
�����鰱���Ƿ��ռ����ķ����ǣ�������������������ͽ� �ۣ�____________________
�ۣ�____________________ ��NH4Cl���������NH3��______________(��ܡ����ܡ�)��
��NH4Cl���������NH3��______________(��ܡ����ܡ�)��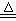 Cl2����MnCl2��2H2O�Իش��������⣺
Cl2����MnCl2��2H2O�Իش��������⣺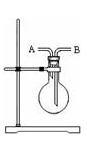
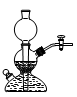
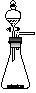
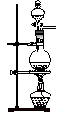
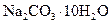 ��������
��������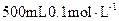 ��
��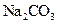 ��Һ����ش��������⡣
��Һ����ش��������⡣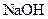 ���壬���������Ƶ�
���壬���������Ƶ�

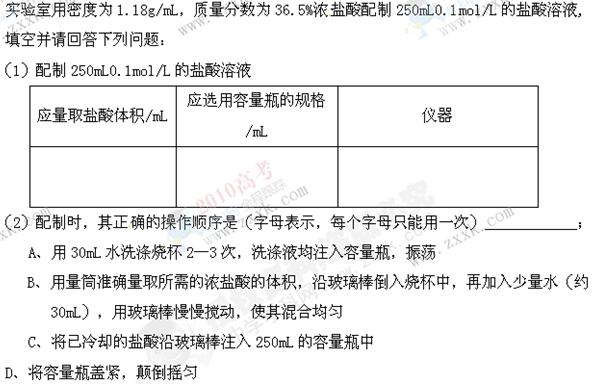
 ��������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��