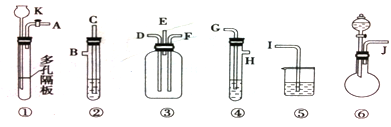
��Ŀ����
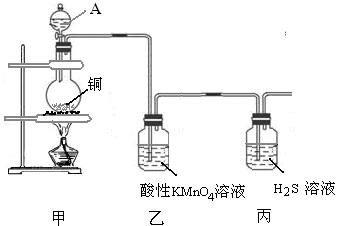
ij��ѧ����С��������ͼװ��̽��SO2�����ʡ�
��ش��������⣺
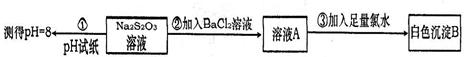
��1��װ�ü���A������������_____________����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
��2��װ�����г��ֵ�������_______________________________������֤��SO2����______������ţ���װ�ñ��з�����Ӧ�Ļ�ѧ����ʽΪ______________________������֤��SO2����______������ţ���
A�������� B����ԭ�� C��Ư����
��3���ռ�SO2�������ѡ���װ��Ϊ______������ţ���
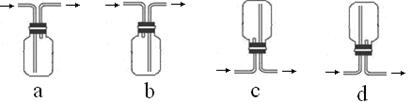
�ӻ����ĽǶȿ��ǣ��ռ�װ�õij�������Ҫ����һ��ʢ��_________���ѧʽ����Һ��ϴ��ƿ��

��ش��������⣺
��1��װ�ü���A������������_____________����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
��2��װ�����г��ֵ�������_______________________________������֤��SO2����______������ţ���װ�ñ��з�����Ӧ�Ļ�ѧ����ʽΪ______________________������֤��SO2����______������ţ���
A�������� B����ԭ�� C��Ư����
��3���ռ�SO2�������ѡ���װ��Ϊ______������ţ���

�ӻ����ĽǶȿ��ǣ��ռ�װ�õij�������Ҫ����һ��ʢ��_________���ѧʽ����Һ��ϴ��ƿ��
��1����Һ©�� Cu+2H2SO4��Ũ�� Cu SO4+SO2��+2H2O��2����Һ��ɫ B
Cu SO4+SO2��+2H2O��2����Һ��ɫ B
2H2S+ SO2=3S��+2H2O a ��3��a c NaOH
 Cu SO4+SO2��+2H2O��2����Һ��ɫ B
Cu SO4+SO2��+2H2O��2����Һ��ɫ B2H2S+ SO2=3S��+2H2O a ��3��a c NaOH
�������������ƿ�з�����Ӧ��Cu+2H2SO4(Ũ)
 CuSO4+ SO2��+2H2O��Cu������ƿ�У�Ũ����ͨ����Һ©�����롣������SO2�����л�ԭ�ԡ������ԡ�Ư���Լ����ԡ�������KMnO4��Һ��֤�仹ԭ�ԣ���������ɫ��dz����ɫ����Ӧ�ķ���ʽΪ��2KMnO4+5SO2+H2O=K2SO4+2MnSO4+2H2SO4 .��H2S��ˮ��Һ����֤�������ԣ������Dz�������ɫ��������Ӧ�ķ���ʽΪ2H2S+ SO2=3S��+2H2O . SO2�����ܽ���ˮ�����Բ�������ˮ���ռ���ֻ�����ſ������ռ������������ܶȱȿ���������ֻ���������ſ������ռ�������ѡ��װ��a��c���ռ���SO2�Ǵ�����Ⱦ�ֱ���ŷŻᵼ�´�����Ⱦ�����꣬������ʵ��װ�õ����װһ��β������װ�ã���������NaOH��Һ������β����2������Ʊ����ռ������ʵ���֤��β��������֪ʶ��
CuSO4+ SO2��+2H2O��Cu������ƿ�У�Ũ����ͨ����Һ©�����롣������SO2�����л�ԭ�ԡ������ԡ�Ư���Լ����ԡ�������KMnO4��Һ��֤�仹ԭ�ԣ���������ɫ��dz����ɫ����Ӧ�ķ���ʽΪ��2KMnO4+5SO2+H2O=K2SO4+2MnSO4+2H2SO4 .��H2S��ˮ��Һ����֤�������ԣ������Dz�������ɫ��������Ӧ�ķ���ʽΪ2H2S+ SO2=3S��+2H2O . SO2�����ܽ���ˮ�����Բ�������ˮ���ռ���ֻ�����ſ������ռ������������ܶȱȿ���������ֻ���������ſ������ռ�������ѡ��װ��a��c���ռ���SO2�Ǵ�����Ⱦ�ֱ���ŷŻᵼ�´�����Ⱦ�����꣬������ʵ��װ�õ����װһ��β������װ�ã���������NaOH��Һ������β����2������Ʊ����ռ������ʵ���֤��β��������֪ʶ��
��ϰ��ϵ�д�
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
�����Ŀ