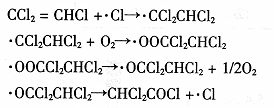��Ŀ����
ij�о���ѧϰС��Թ���̿������������Ӧ���������ijɷֽ������о���
��������衿 �÷�Ӧ�е�������������CO������CO2��CO�Ļ���
���������ϡ� ��������̼��������������Ӧ��ʵ���ҿ������Ȼ�隣�����Һ���������ƣ�NaNO2��������Һ��ϼ��ȷ�Ӧ�Ƶõ�����
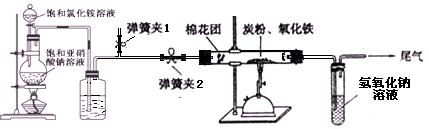
����Ʒ����� ��ͼ��ʾ����һ�������������ڸ��������������������̿�۳�ַ�Ӧ���ⶨ�μӷ�Ӧ��̼Ԫ������Ԫ�ص������ȡ�
�Իش��������⣺
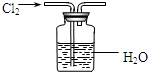
(1) ����ͼ����װ�ã���μ���װ�õ�����
�� ��
(2) ���ƿ��ʢ�ŵ��Լ�Ϊ ��������
Ϊ ��
(3) ʵ�鿪ʼʱ��Ӧ�ȴ��ɼ�2һ��ʱ���رգ�ͬʱ���ɼ�1���ٵ�ȼ�ƾ���ƣ�������
�� ��
(4) ��ȡ3.20 g��������2.00 g̿�ۻ�Ͼ��ȣ���������Ϊ48.48 g��Ӳ�ʲ������У�����Ӧ��������ͨһ��ʱ��ĵ�������ȴ�����£��Ƶ�Ӳ�ʲ����ܺ���������Ϊ52.24 g������һ���ⶨ��֪�μӷ�Ӧ����Ԫ������Ϊ0.96 g���Ӷ�ȷ�ϸ÷�Ӧ�����������CO2��CO�Ļ��������� ���������ݴ�������жϣ���Ӧ������������n��CO2��: n��CO���� ��
(5) ��ͬѧ����ʵ��ó��Ľ��ۣ���ΪӦ��ʵ��װ�ý�һ�����ƣ�����ΪӦ����θĽ���
��
��������衿 �÷�Ӧ�е�������������CO������CO2��CO�Ļ���
���������ϡ� ��������̼��������������Ӧ��ʵ���ҿ������Ȼ�隣�����Һ���������ƣ�NaNO2��������Һ��ϼ��ȷ�Ӧ�Ƶõ�����
����Ʒ����� ��ͼ��ʾ����һ�������������ڸ��������������������̿�۳�ַ�Ӧ���ⶨ�μӷ�Ӧ��̼Ԫ������Ԫ�ص������ȡ�
 |
�Իش��������⣺
(1) ����ͼ����װ�ã���μ���װ�õ�����
�� ��
(2) ���ƿ��ʢ�ŵ��Լ�Ϊ ��������
Ϊ ��
(3) ʵ�鿪ʼʱ��Ӧ�ȴ��ɼ�2һ��ʱ���رգ�ͬʱ���ɼ�1���ٵ�ȼ�ƾ���ƣ�������
�� ��
(4) ��ȡ3.20 g��������2.00 g̿�ۻ�Ͼ��ȣ���������Ϊ48.48 g��Ӳ�ʲ������У�����Ӧ��������ͨһ��ʱ��ĵ�������ȴ�����£��Ƶ�Ӳ�ʲ����ܺ���������Ϊ52.24 g������һ���ⶨ��֪�μӷ�Ӧ����Ԫ������Ϊ0.96 g���Ӷ�ȷ�ϸ÷�Ӧ�����������CO2��CO�Ļ��������� ���������ݴ�������жϣ���Ӧ������������n��CO2��: n��CO���� ��
(5) ��ͬѧ����ʵ��ó��Ľ��ۣ���ΪӦ��ʵ��װ�ý�һ�����ƣ�����ΪӦ����θĽ���
��
(1) ������װ��ĩ�˽�һ���䲣�����ܵ��齺�ܣ�������������ĩ�˷���ʢ��ˮ��ˮ���У��þƾ��������巢��װ�������ȣ���������ĩ�������ݲ�����ֹͣ���Ⱥ�������������һ��ˮ������©��
(2) Ũ���ᣬ���ղ����ĵ����е�ˮ����ø���ĵ�����
(3) Ϊ���ž�����װ���еĿ������رյ��ɼ�1�����ɼ�2�������Ƶ���װ������ѹ���ߣ����ܻ��з�����ը��Σ�ա�
(4) �����㷴Ӧ���������̼����ԭ�ӵ����ʵ���֮��Ϊ2��3��1��1��
(5) ��β�����ڴ���һ��ȼ�ľƾ��ƻ�����һβ������װ��
(2) Ũ���ᣬ���ղ����ĵ����е�ˮ����ø���ĵ�����
(3) Ϊ���ž�����װ���еĿ������رյ��ɼ�1�����ɼ�2�������Ƶ���װ������ѹ���ߣ����ܻ��з�����ը��Σ�ա�
(4) �����㷴Ӧ���������̼����ԭ�ӵ����ʵ���֮��Ϊ2��3��1��1��
(5) ��β�����ڴ���һ��ȼ�ľƾ��ƻ�����һβ������װ��
��ʵ��ɹ��Ĺؼ��Dz����е�̼Ԫ��ȫ������̿�ۡ���Ԫ��ȫ��������������Ҫ�ų�������������������̼��ʵ��ĸ��ţ����õ����ž�װ���еĿ���������Ӳ�ʲ������������ļ��������������Һ������ȷ������ijɷ֡�
(1) ��ĩ�˲����ܽ���ʢ��ˮ���ձ����ƾ��������巢��װ�ÿ��Ƿ�������ð����������ͬѧ�������뵽������װ�����ܣ���ȴ��װ�������������С������������ˮ������������������ͬѧ�����Եġ�
(2) �������ˮҲ�ᱻ����������Һ���գ�����ʵ�飬������ﵪ����
(3) Ϊ���ž�����װ���еĿ������رյ��ɼ�1�����ɼ�2�������Ƶ���װ������ѹ���ߣ����ܻ��з�����ը��Σ�ա�
(4) Ӳ�ʲ����ܼ���1.44�ˣ�������0.96�ˣ���̼��0.48�ˡ��μӷ�Ӧ��̼����ԭ������Ϊ2��3���ʿɵó����ۡ���n��CO2��= x��n��CO��=y����x+y���� (2x+y)=2��3,x��y=1��1��
(5) β���к����ж�����CO������ȼ��������Ⱦ������
(1) ��ĩ�˲����ܽ���ʢ��ˮ���ձ����ƾ��������巢��װ�ÿ��Ƿ�������ð����������ͬѧ�������뵽������װ�����ܣ���ȴ��װ�������������С������������ˮ������������������ͬѧ�����Եġ�
(2) �������ˮҲ�ᱻ����������Һ���գ�����ʵ�飬������ﵪ����
(3) Ϊ���ž�����װ���еĿ������رյ��ɼ�1�����ɼ�2�������Ƶ���װ������ѹ���ߣ����ܻ��з�����ը��Σ�ա�
(4) Ӳ�ʲ����ܼ���1.44�ˣ�������0.96�ˣ���̼��0.48�ˡ��μӷ�Ӧ��̼����ԭ������Ϊ2��3���ʿɵó����ۡ���n��CO2��= x��n��CO��=y����x+y���� (2x+y)=2��3,x��y=1��1��
(5) β���к����ж�����CO������ȼ��������Ⱦ������

��ϰ��ϵ�д�
�����Ŀ