��Ŀ����
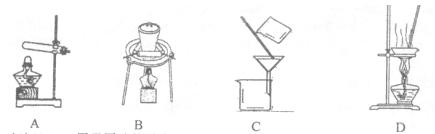
��1��ʵ�����ɴ�����ͭм�Ƶ�����CuSO4��5H2O����ʵ���������£�
�ܽ�ͭмһ�ַ����ǣ���ͭм���뵽ϡ������˫��ˮ�Ļ��Һ�в���30��40��ˮԡ���ȣ�һ��ʱ���ͭ��ȫ�ܽ⣬�õ�����ͭ��Һ��
�ٸ÷�Ӧ�Ļ�ѧ����ʽΪ ��
�ڷ�Ӧ�¶Ȳ��ܳ���40���ԭ���� ��
��������ͭ��Һ��õ����IJ�������Ϊ ����ȴ�ᾧ�� ��ϴ�ӡ����
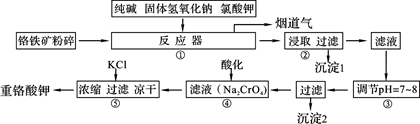
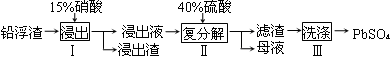
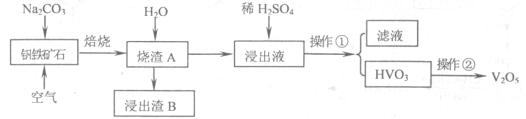
��2��Ŀǰ�ҹ��Ļ����������������Ϊȼú���飬����ȼúΪ���ĵ�����������ɵĻ�����Ⱦ����Լ������ҵ��չ��һ����Ҫ���أ����е������NOx���Ǽ̷۳��Ͷ�������֮��ȼú��վ�����������ص㡣
��ȼú��������ķ����ܶ࣬��ʯ��ʯ��ʯ�෨����ˮ���ȡ�����ʯ��ʯ-ʯ�෨�����ԭ����һ����SO2+Ca(OH)2=CaSO3+H2O��Ȼ���ٽ����������Ƴ�ʯ��,д���÷�Ӧ�Ļ�ѧ����ʽ ��
��ȼú���������ɲ��ð���NH3����Ϊ��ԭ���ʣ��ڴ������ڵ������£���������(NOx)�뻹ԭ��������Ӧ���������ĵ�����ˮ��д�����������백��Ӧ�Ļ�ѧ����ʽ ��
��1����H2SO4+H2O2+Cu=CuSO4+2H2O;
�ڷ�ֹ��������ķֽ⣻
������Ũ��������
��2����2CaSO3+O2=2CaSO4������CaSO4��H2O��CaSO4��2H2O��ƽҲ���֣�
��6NO2+8NH3=7N2+12H2O
���������������1����ͭ��ϡ���ᡢ˫��ˮ����������ԭ��Ӧ����������ͭ��Һ����ѧ����ʽΪH2SO4+H2O2+Cu=CuSO4+2H2O;
�ڹ������ⲻ�ȶ��������ֽ⣬���Է�Ӧ�¶Ȳ��ܳ���40��
������Һ�õ�����IJ���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����
��2����ʯ��Ϊ����ƵĽᾧˮ������������������Ӧ�ɵ�ʯ�࣬��ѧ����ʽΪ2CaSO3+O2=2CaSO4
�ڶ��������백��Ӧ�������ĵ�����ˮ,��ѧ����ʽΪ6NO2+8NH3=7N2+12H2O
���㣺����Ի�ѧʵ��ķ������������жϣ���ѧ����ʽ����д

 �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д� �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д����Խ�������ɫҺ�壺C2H5OH��AgNO3��Һ��C2H5Br��KI��Һ��C6H5OH��Һ��C6H6���ֿ����Լ���
| A��FeCl3��Һ | B��ϡ���ᡡ |
| C�����Ը��������Һ | D��NaOH��Һ |
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ� Fe2����Mg2����Al3����Ba2����NO3-��SO42-��Cl����I����HCO3-��ȡ����Һ����ʵ�飺
| ʵ�鲽�� | ʵ������ |
| (1)ȡ��������Һ���Ӽ�����ɫʯ����Һ | ��Һ��� |
| (2)ȡ��������Һ���ȣ���CuƬ��ŨH2SO4������ | ����ɫ���������������������ɺ���ɫ |
| (3)ȡ��������Һ����BaCl2��Һ | �а�ɫ���� |
| (4)ȡ(3)���ϲ���Һ����AgNO3��Һ | �а�ɫ�������Ҳ�����ϡHNO3 |
| (5)ȡ��������Һ����NaOH��Һ | �а�ɫ������NaOH����ʱ���������ܽ� |
�ɴ��жϣ�
(1)��Һ�п϶������ڵ�������________����Һ�п϶����ڵ�������____��
(2)�����ʵ����֤���п��ܴ��ڵ������ӵķ���(д��������������)_____��
 2RAn(�л���)+nH2SO4(ˮ��)��
2RAn(�л���)+nH2SO4(ˮ��)��