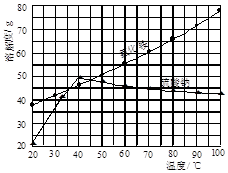��Ŀ����
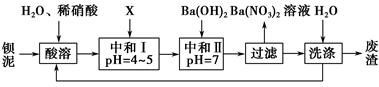
��ҵ�ϳ��ø�������Ч�ɷ�ΪFeO��Cr2O3����Ҫ����ΪSiO2��Al2O3��Ϊԭ�������ظ���أ�K2Cr2O7����ʵ����ģ�ҵ���ø��������ظ���ص���Ҫ������������ͼ���漰����Ҫ��Ӧ�ǣ�6FeO��Cr2O3��24NaOH��7KClO3=12Na2CrO4��3Fe2O3��7KCl��12H2O���Իش��������⣺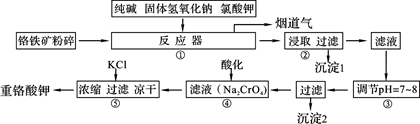
��1��������Һ���������ӵļ��鷽���� ��
��2������۱�����������Ϊ�������ӷ��ţ� ��
��3���ڷ�Ӧ�����У����������봿�Ӧ�Ļ�ѧ����ʽΪ�� ��
��4���̵����е�CO2����H2�ϳɼ״���CH3OH��H2��ȼ���ȷֱ�Ϊ����H=��725.5 kJ/mol����H=��285.8 kJ/mol��д����ҵ����CO2��H2�ϳ�CH3OH���Ȼ�ѧ����ʽ�� ��
��5��2011�����������ĸ���Ⱦ�¼���˵��������������ˮ���������ŷŶ��������滷���м����Σ������ⷨ�Ǵ�������Ⱦ��һ�ַ�������������������ʯī��������⺬Cr2O72-�����Է�ˮ��һ��ʱ������Fe(OH)3��Cr(OH)3������
��д����ⷨ������ˮ���ܷ�Ӧ�����ӷ���ʽ ��
����֪Cr(OH)3��Ksp=6.3��10�C31�����ر�ˮ�����������ֵ��0.1 mg/L��Ҫʹ��Һ��c(Cr3+)�������ϵر�ˮ��ֵ���������Һ��c(OH-)�� mol/L��ֻд�������ʽ����
��1����ɫ��Ӧ ����2��SiO32-��AlO2-��
��3��Na2CO3��SiO2 Na2SiO3��CO2����
Na2SiO3��CO2����
��4��CO2(g)��3H2(g) = CH3OH(l)��H2O(l) ��H=��131.9 kJ/mol��
��5���� 6Fe��Cr2O72-��2H+��17H2O = 6Fe(OH)3����2Cr(OH)3����6H2����
�� ����
���� ��
��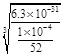 ����
����
���������������1��������Һ����������K+�ļ��鷽��������ɫ��Ӧ�����顣������ȡ����������˿��Pt˿��������ϴ�Ӻ��ھƾ��ƵĻ��������գ�����������ɫ��ͬʱպȡ����Һ���ڻ��������գ�������ɫCo�������۲�������ɫ����������ɫ����֤������K+����2���������������NaOH��Na2CO3�ȼ������ʣ�SiO2��Al2O3������Ӧ��ΪNa2SiO3��AlO2������������Һ��pH��7��8ʱ����ת��ΪH2SiO3��Al(OH)3��������˲���۱�����������ΪSiO32-��AlO2-����3���ڷ�Ӧ�����У����������봿�Ӧ�Ļ�ѧ����ʽΪ��Na2CO3��SiO2 Na2SiO3��CO2����(4)CH3OH��H2��ȼ�յ��Ȼ�ѧ����ʽΪ���� CH3OH(l)+3/2O2(g)=CO2(g)+2H2O(l) ��H=��725.5 kJ/mol ; ��H2(g)+ 1/2O2(g)=H2O(l) ��H=��285.8 kJ/mol. �ڡ�3���١������ɵ�CO2(g)��3H2(g) = CH3OH(l)��H2O(l) ��H=��131.9 kJ/mol����5���ٸ��ݵ����غ㡢����غ㼰�����غ㶨�ɿɵõ�⺬Cr2O72-�����Է�ˮ���ܷ�Ӧ�����ӷ���ʽΪ��6Fe��Cr2O72-��2H+��17H2O = 6Fe(OH)3����2Cr(OH)3����6H2���������ر�ˮ�����������ֵ��0.1 mg/L ��c(Cr3+) =1��10-4g��52g/mol/L=
Na2SiO3��CO2����(4)CH3OH��H2��ȼ�յ��Ȼ�ѧ����ʽΪ���� CH3OH(l)+3/2O2(g)=CO2(g)+2H2O(l) ��H=��725.5 kJ/mol ; ��H2(g)+ 1/2O2(g)=H2O(l) ��H=��285.8 kJ/mol. �ڡ�3���١������ɵ�CO2(g)��3H2(g) = CH3OH(l)��H2O(l) ��H=��131.9 kJ/mol����5���ٸ��ݵ����غ㡢����غ㼰�����غ㶨�ɿɵõ�⺬Cr2O72-�����Է�ˮ���ܷ�Ӧ�����ӷ���ʽΪ��6Fe��Cr2O72-��2H+��17H2O = 6Fe(OH)3����2Cr(OH)3����6H2���������ر�ˮ�����������ֵ��0.1 mg/L ��c(Cr3+) =1��10-4g��52g/mol/L= ��10-4 mol/L .Cr(OH)3��Ksp=6.3��10�C31����c(Cr3+)��c3(OH-)��6.3��10�C31;
��10-4 mol/L .Cr(OH)3��Ksp=6.3��10�C31����c(Cr3+)��c3(OH-)��6.3��10�C31;
c3(OH-)��6.3��10�C31�� ��10-4=6.3��52��10-27.����c(OH-)=
��10-4=6.3��52��10-27.����c(OH-)= .
.
���㣺�������ӵļ��顢��������ѧ����ʽ���Ȼ�ѧ����ʽ����д��������ȥ��������ʱ��Һ��pH�ļ��㡣

��ȥCO2�е�HCl��������ǽ��������ͨ��������
| A������NaOH��Һ | B������NaHCO3��Һ |
| C������Na2CO3��Һ | D��ˮ |
����ƹ㷺����ʳƷ��������ʯ�͵ȹ�ҵ�����ϣ�300��400�����ҷֽ⡣��ʵ������ȡ�ķ���֮һ�ǣ�Ca(OH)2 +2HCHO + H2O2 = Ca(HCOO)2 + 2H2O + H2����
ʵ������ȡʱ������ҵ���������ƺͼ�ȩ���μ��뵽��������Ϊ30-70%�Ĺ���������Һ�У�Ͷ�����ʵ���֮������Ϊ1��2��1.2�������տɵõ���������98%�������ؽ����������͵����ʲ�Ʒ��
��1��������������������Զ࣬��Ŀ���� ��
��2����Ӧ�¶���ÿ�����30-70��֮�䣬�¶Ȳ����ߣ�����Ҫԭ���� ��
��3���Ʊ�ʱ�ڻ����Һ��Ҫ���������������Ƽ�ȩ��������Ӧ�⣬��Ҫ����������Na2S��Һ�������Ƶ�Ŀ���� ��
��4��ʵ��ʱ��ǿ������45min����Ŀ���� ���������������Һ��pH 7��8����Ŀ���� ����ᾧ���롢����ò�Ʒ��
��ij�о���ѧϰС���ù�ҵ̼���(��Ҫ�ɷ�ΪCaCO3������Ϊ��Al2O3��FeCO3) Ϊԭ�ϣ����Ʊ������Σ������������Һ�����ȡ����ơ������ͼ�������ʵ��ܽ������������ؽ������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0 mol��L-1����)�����ṩ���Լ��У�a.�����ƣ�b.5mol��L��1���ᣬc. 5mol��L��1���ᣬd. 5mol��L��1���ᣬe. 3%H2O2��Һ��f.����ʯ��ˮ��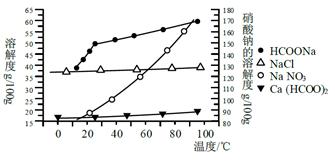
�벹��������̼����Ʊ�����Ƶ�ʵ�鲽��
| ���� ���� | ��ʼ���� ��pH | ������ȫ ��pH |
| Fe3�� | 1. 1 | 3. 2 |
| Al3�� | 3. 0 | 5. 0 |
| Fe2�� | 5. 8 | 8. 8 |
����1.��ȡ13.6g����������Լ20mLˮ������ܴ��ã�����ȡ��ϸ��̼�����Ʒ10g���á�
����2. ��
����3. ��
����4.���˺���Һ���������Һ��ϣ�������ҺpH 7��8����ֽ��裬������Һ������Ũ���� ��ϴ�ӡ�60��ʱ����ü���ƾ��塣
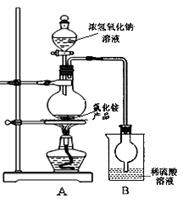
�����������ѧ���õ��Լ�����ҵ�������̿��Ʊ���������������¡�
��1��д��ʵ��������KMnO4�ֽ���ȡO2�Ļ�ѧ����ʽ
��2��KMnO4ϡ��Һ��һ�ֳ��õ���������������ԭ��������������ͬ����
| A��84����Һ(NaClO��Һ) |
| B��˫��ˮ |
| C������ |
| D��75%�ƾ� |
��4��д����Ӧ�ٵĻ�ѧ����ʽ
��5��������������� �����������KMnO4��K2CO3�������� ����
���ʣ��ϵIJ��죬���� ����������裩�����ȹ��˵õ�KMnO4�־��塣
��6�����������п���ѭ��ʹ�õ������� �� ��д��ѧʽ��,���ڴ�����100�����̿�MnO287.0%���������Ͽ�����KMnO4���� �֣��������Ʊ�������ԭ�ϵ���ʧ����
��ҵ�����õ�����ࣨ��Ҫ����Fe2O3��CuO��Cr2O3�������������ʣ�����ͭ���Ƚ����������������£�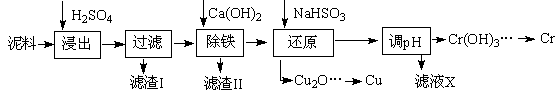
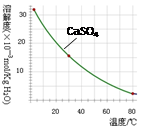
��֪�������ʳ�����pH��CaSO4���ܽ���������£�
| | Fe3+ | Cu2+ | Cr3+ |
| ��ʼ����pH | 2��1 | 4��7 | 4��3 |
| ��ȫ����pH | 3��2 | 6��7 | a |
��1���ڽ��������г�������Fe2(SO4)3��Cr2(SO4)3��,��Ҫ����
��2���ڳ��������У���Ҫ��ȥFe3+��CaSO4,�������ز������ټ���ʯ�������pH�� ���ڽ���Һ���ȵ�80�棬 ��
��3��д����ԭ�����м���NaHSO3����Cu2O��������ӷ�Ӧ����ʽ ���˲����м���NaHSO3�õ�Cu2O�IJ���Ϊ95%����NaHSO3�����������˷��Լ��⣬������ֵ������� ��
��4��������Ũ�ȡ�1��10��5 mol?L-1��Ϊ������ȫ����ҪʹCr3+��ȫ������Ҫ����C(OH��)�� ����֪��Ksp[Cr(OH)3]=6��3��10-31��
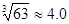 ��
�� ��ͼ�ǹ���ҩ�ﻪ��Ƭ(���ص�Ƭ)��ʹ��˵���飬����Ϊ˵����IJ������ݣ�
| ����Ƭ(���ص�Ƭ)ʹ��˵���� ��Ʒ������ɡ� Ʒ�������ص�Ƭ ��ɣ�����Ƭ Ӣ������Cydiodine Tablets ����Ƭ(���ص�Ƭ)����Ҫ���Գɷ��Ƿ��ӵ⣬����1.5 mg/Ƭ���������÷��ӷ�ɢ�����Ƴɷ���̬���ص⣬�������������Ե� �����ء� �ڹ⡢�ܱա����������� ����Ч�ڡ� ���� |
(1)��������˵������ѧ֪ʶ�ش�
�ٻ���Ƭ�к��еĻ��Գɷ��� (д����ʽ)��
�����ƶϻ���Ƭ (��ǡ����ǡ�)��ɫ��
(2)ijѧ��Ϊ��֤����Ƭ��ȷʵ���������ɷ֣����ʵ�����¡�����գ�
��ȡһ��ҩƬ�����в������飬�ٽ�ҩ��װ���Թܲ�����Լ2 mL����ˮ�����Թ����ټ���Լ2 mL (��ѡ����ĸ)����������
A���ƾ�(�ܶȱ�ˮС����ˮ������Ȼ���)
B�����Ȼ�̼(�ܶȱ�ˮ������ˮ)
�����������Һ�����ܹ۲쵽������ ��
��ѡ�ø�Һ���ԭ���� ��
(3)���������һ�ַ�������֤����Ƭ�еijɷ�(������ʵ��ԭ��������������ʵ�����) ��