��Ŀ����
����Ŀ�������ס����Ԫ�ؼ��仯�������ִ�ũҵ���Ƽ�����������������������ص���;��
�Ż�̬��ԭ����������ߵ��ܼ�Ϊ________�������йر�ʾ��̬��ԭ�ӵ��Ų�ͼ�У���Υ�����ع������________��
A.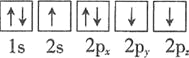
B.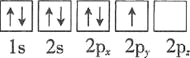
C.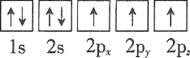
D.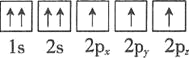
������һ�����õĻ������ȼ�ϣ���ͳ�Ʊ��µķ�����NaClO + 2NH3 = N2H4 + NaCl + H2O����֪�µ��۵㡢�е�ֱ�Ϊ1.4�桢113.5�棬�������۵㡢�е�ֱ�Ϊ-77.7�桢-33.5�档
�ٵ�������������Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ________��
��N2H4�е�ԭ�ӵ��ӻ��������Ϊ________��H2O�ķ��ӹ���Ϊ________��
��NH3��H2O����������ԭ���ӻ�������ͬ����ˮ�����м��DZ�NH3�еļ���С����ԭ����________________________________________���������백���۵㡢�е��������Ҫ��ԭ����______________________________________________________________��
�������뵼�������֮���黯�ؾ����У�As��Gaԭ��������Ӳ���ﵽ8�����ȶ��ṹ����þ����еĻ�ѧ����������________��
A. ���Ӽ� B. ���Լ� C. ��λ�� D. ![]() ��
��
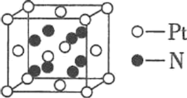
���ɵ�����(Pt)��Ԫ���γɵ�ij�ֶ�Ԫ������ľ�����ͼ��ʾ����û�����Ļ�ѧʽ________�����þ����ı߳�Ϊd pm����þ�����ܶ�Ϊ________ gcm-3��
���𰸡�4p B N > O > Na sp3 V�� ˮ��������2���µ��Ӷԣ���NH3��ֻ��һ���µ��Ӷԣ�ǰ�߶Գɼ������ų�������ǿ N2H4���Ӽ������Ŀ����NH3���Ӽ������Ŀ BC PtN2 ![]()
��������
�Ż�̬��ԭ����������ߵ��ܼ�Ϊ4p�����ع��������ȵ���ռ��һ�������
�Ƣٸ��ݵ�һ�����ܵĹ��ɿ��Եó���һ�����ܣ���N2H4������Nԭ�ӳ��γ�3���������һ�Թµ��ӣ�H2O�ķ����������Թµ��ӣ���ˮ��������2���µ��Ӷԣ���NH3��ֻ��һ���µ��Ӷԣ�ǰ�߶Գɼ������ų�������ǿ��N2H4���Ӽ������Ŀ����NH3���Ӽ������Ŀ��
��Gaԭ���������3�����ӣ�As��5�����ӣ�Ҫ��8�����ȶ��ṹ�������γ���λ����
���þ�̯����֪������Pt��N�����������ܶȼ��㹫ʽ���㡣
�Ż�̬��ԭ����������ߵ��ܼ�Ϊ4p��ֻ��B��Υ�����ع��ʴ�Ϊ��4p��B��
�Ƣٸ��ݵ�һ�����ܵĹ��ɿ��Եó�����һ�����ܣ�N > O > Na���ʴ�Ϊ��N > O > Na��
��N2H4������Nԭ�ӳ��γ�3���������һ�Թµ��ӣ����ӻ���ʽΪ��sp3��H2O�ķ����������Թµ��ӣ��ʷ��ӹ����ǣ�V�Σ��ʴ�Ϊ��sp3��V�Ρ�
��ˮ�����м��DZ�NH3�еļ���С����ԭ���ǣ�ˮ��������2���µ��Ӷԣ���NH3��ֻ��һ���µ��Ӷԣ�ǰ�߶Գɼ������ų�������ǿ���������백���۵㡢�е��������Ҫ��ԭ���ǣ�N2H4���Ӽ������Ŀ����NH3���Ӽ������Ŀ���ʴ�Ϊ��ˮ��������2���µ��Ӷԣ���NH3��ֻ��һ���µ��Ӷԣ�ǰ�߶Գɼ������ų�������ǿ��N2H4���Ӽ������Ŀ����NH3���Ӽ������Ŀ��
��Gaԭ���������3�����ӣ�As��5�����ӣ�Ҫ��8�����ȶ��ṹ�������γ���λ������ѡBC���ʴ�Ϊ��BC��
���þ�̯����֪�����к���4��Pt��8��N���ʻ�ѧʽ�ǣ�PtN2���ֿ��Ը����ܶȼ��㹫ʽ��֪���ܶ�Ϊ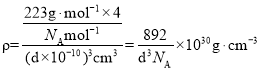 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

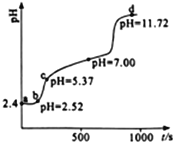
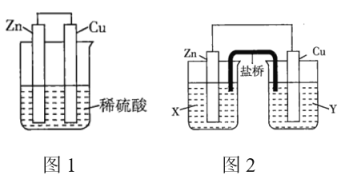
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�����Ŀ�����ǵ����Ϻ����ḻ��һ��Ԫ�أ���Ԫ�صĵ��ʼ��仯�����ڹ�ũҵ������������������Ҫ���á�
�Ÿ������������仯ʾ��ͼ����д�� NO �� CO2��Ӧ���Ȼ�ѧ����ʽ_________��
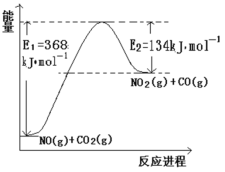
���ڹ̶�������ܱ������У��������»�ѧ��Ӧ��2NH3(g)![]() N2(g)+3H2(g) ��H��0����ƽ�ⳣ�� K ���¶� T �Ĺ�ϵ���±���
N2(g)+3H2(g) ��H��0����ƽ�ⳣ�� K ���¶� T �Ĺ�ϵ���±���
T/K | 298 | 398 | 498 |
ƽ�ⳣ��K | 4.1��106 | K1 | K2 |
�����ж� K1_________K2����д��������������������������
���÷�Ӧ���ر�S_________0���� >��< �� = ��
�����и�����˵���÷�Ӧ�Ѵﵽƽ��״̬����_________������ĸ����
a��������N2��H2��NH3�����ʵ���֮��Ϊ1��3��2 b��2��(NH3)�� �� 3��(H2)��
c�������ڻ������ƽ����Է����������ֲ��� d�����������ܶȱ��ֲ���
��һ���¶��£��� 1L �ܱ������г��� 1molN2�� 3molH2 ��������Ӧ���������ݻ��㶨��10min �ﵽƽ��ʱ������������ʵ���Ϊԭ����9/10����N2��ת����![]() (N2)=_________________
(N2)=_________________
�ǶԷ�Ӧ N2O4��g��![]() 2NO2��g�� ��H > 0 �����¶ȷֱ�ΪT1��T2 ʱ��ƽ����ϵ�� NO2�����������ѹǿ�仯��������ͼ��ʾ������˵����ȷ����__________��
2NO2��g�� ��H > 0 �����¶ȷֱ�ΪT1��T2 ʱ��ƽ����ϵ�� NO2�����������ѹǿ�仯��������ͼ��ʾ������˵����ȷ����__________��
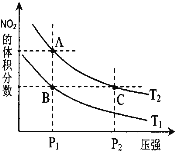
a��A��C ����ķ�Ӧ���ʣ�A��C
b��A��C ����Ļ�ѧƽ�ⳣ����A��C
c��A��C ���� N2O4��ת���ʣ�A��C
d����״̬ B ��״̬ A�������ü��ȵķ���
����Ŀ�������£���һ��Ũ�ȵ�HA��HB�ֱ���0.10mol/L��NaOH��Һ�������ϣ�ʵ���¼���±���
ʵ���� | ������� | �������Ũ��/(mol/L) | ��Ϻ���Һ��pH |
�� | HA | 0.10 | 8.7 |
�� | HB | 0.12 | 2 |
����˵������ȷ����
A. HA��ǿ�ᣬHB������
B. �����¶ȣ���Һ����c(B-)/c(Na+������
C. ��Һ��������Ũ�ȵĹ�ϵ��c(A-)>c(Na+)>c(OH->c(H+��
D. ��Һ��������Ũ�ȵĹ�ϵ��c(Na+)��c(H+��c(B-)=0.12 mol/L