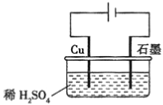
��Ŀ����
����Ŀ��A��B��C��D��E��F��GΪԭ��������������Ķ���������Ԫ�ء�B��C��D������A�γ�10���ӷ��ӣ�E���ʿ����ں��Ӹֹ죬F��Dͬ���壬F��Gͬ���ڡ�
��1��D��E��F �����Ӱ뾶�ɴ�С��˳��Ϊ_________(�����ӷ���)��
��2��д����֤��G��F�ǽ�����ǿ��һ�����ӷ���ʽ_____________��
��3��F��G��һ�ֻ������������ԭ�Ӿ�Ϊ8�����ȶ��ṹ���û�������ˮ��Ӧ����F���ʡ�F����ۺ������G���⻯����ֲ�������ʵ���֮��Ϊ2:1:6,�ĵ���ʽΪ_______���÷�Ӧ�Ļ�ѧ����ʽΪ___________________��
��4��C�ֱܷ���A��D��ԭ�Ӹ�����1:2�γɻ������Һͱ����ҵĽṹʽΪ_______��������,Һ�������������Ӧ������������Ⱦ�����ʣ���������1mol����ʱ����QkJ,�÷�Ӧ���Ȼ�ѧ����ʽΪ_____________________��
��5����ȡ100mL 1mol/L��E���Ȼ�����Һ,�����м���1mol/L ����������Һ������3.9g����������������������Һ�������Ϊ_________mL��
���𰸡� S2->O2->Al3+ Cl2+H2S=2H++2Cl-+S��(��Cl2+S2-=2Cl-+S��) ![]() 3SCl2+4H2O=2S+H2SO4+6HCl
3SCl2+4H2O=2S+H2SO4+6HCl ![]() 2N2H4(1)+2NO2(g)=3N2(g)+4H2O(l)��H=-7QkJ/mol 150��350
2N2H4(1)+2NO2(g)=3N2(g)+4H2O(l)��H=-7QkJ/mol 150��350
�����������������A��B��C��D��E��F��GΪԭ��������������Ķ���������Ԫ�ء�B��C��D������A�γ�10���ӷ��ӣ� F��Dͬ���壬F��Gͬ���ڣ�����A��HԪ����B��C��D �ǵڶ�����Ԫ�أ�F��G�ǵ�������Ԫ�أ�B��̼Ԫ�ء�C�ǵ�Ԫ�ء�D����Ԫ�أ�F����Ԫ�ء�G����Ԫ�أ�E���ʿ����ں��Ӹֹ죬E����Ԫ�ء�
��������1�����Ӳ���Խ��뾶Խ���Ӳ�����ͬʱ��������Խ��뾶ԽС��O2-��Al3+��S2- �İ뾶�ɴ�С��˳��ΪS2->O2->Al3+��
��2���ǽ�����Խǿ������������Խǿ��Cl2+H2S=2H++2Cl-+S����֤��Cl��S�ǽ�����ǿ��
��3��Cl��S��һ�ֻ��������ˮ��Ӧ����S���ʡ�H2SO4��HCl�����ֲ�������ʵ���֮��Ϊ2:1:6������Ԫ���غ㣬�Ļ�ѧʽ��SCl2������ʽΪ![]() ���÷�Ӧ�Ļ�ѧ����ʽΪ3SCl2+4H2O=2S+H2SO4+6HCl��
���÷�Ӧ�Ļ�ѧ����ʽΪ3SCl2+4H2O=2S+H2SO4+6HCl��
��4��N�ֱܷ���H��O��ԭ�Ӹ�����1:2�γɻ������Һͱ�������N2H4������NO2��N2H4�ĽṹʽΪ![]() ��������,Һ��N2H4��NO2���巴Ӧ�������ֵ�����ˮ����������1mol����ʱ����QkJ,�÷�Ӧ���Ȼ�ѧ����ʽΪ2N2H4(1)+2NO2(g)=3N2(g)+4H2O(l) ��H=-7QkJ/mol��
��������,Һ��N2H4��NO2���巴Ӧ�������ֵ�����ˮ����������1mol����ʱ����QkJ,�÷�Ӧ���Ȼ�ѧ����ʽΪ2N2H4(1)+2NO2(g)=3N2(g)+4H2O(l) ��H=-7QkJ/mol��
��5��3.9g�����������������ʵ�����0.05mol����������ֻ��������������������Ҫ����������Һ�������VL
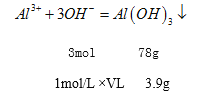
![]() ��V=0.15L=150mL��
��V=0.15L=150mL��
��������������������������ƫ���������������������������������������150mL������ƫ�����Ƶ����ʵ�����0.1L�� 1mol/L��0.05mol=0.05mol������ƫ������������������0.05mol ��4=0.2mol����������������Һ�����Ϊ0.2mol��1mol/L=0.2L=200mL������������������Һ��Ϊ350 mL��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�������Ļ�����㷺��������Ȼ�磬��һ��dz���Ҫ�Ļ�����ش���������:
��1����һ��������:2N2(g)+6H2O(g)=4NH3(g)+3O2(g)����֪�÷�Ӧ����صĻ�ѧ��������������:
��ѧ�� | N��N | H-O | N-H | O=O |
E/(kJ/mol) | 946 | 463 | 391 | 496 |
��÷�Ӧ����H=________kJ/mol.
��2���ں����ܱ������г���2molNO2��1molO2������Ӧ����:4NO2(g)+O2(g)![]() 2N2O5(g)
2N2O5(g)
����֪�ڲ�ͬ�¶��²��N2O5�����ʵ�����ʱ��ı仯��ͼ1��ʾ����������,�÷�Ӧ�������Է����У�ԭ����___________________________��

�������йظ÷�Ӧ��˵����ȷ����________��
A.�������������ƽ�����淴Ӧ�����ƶ������������ɫ����
B.���º���,�ٳ���2molNO2��1molO2,�ٴδﵽƽ��ʱNO2ת��������
C.���º��ݣ��������ڵ��ܶȲ��ٸı䣬��Ӧ�ﵽƽ��״̬
D.���÷�Ӧ��ƽ�ⳣ��������һ���ǽ������¶�
��3��N2O5��һ��������ɫ������,���Ʊ����������⻯��ȼ�ϵ������Դ�����õ�ⷨ�Ʊ��õ�N2O5,����ԭ����ͼ�������⻯��ȼ�ϵ�صĸ�����ӦʽΪ___________��

��4��X��Y��Z��W�ֱ���HNO3��NH4NO3��NaOH��NaNO2����ǿ������е�һ�֡��ϱ��dz�����Ũ�Ⱦ�Ϊ0.01mol/L��X��Y��Z��W��Һ��pH����X��Y��Z��1molͬʱ����ˮ�еõ������Һ��������Һ�и����ӵ�Ũ���ɴ�С��˳��Ϊ________________��
��5�������������������ڴ����еĺ������������ʱ���漰���·�Ӧ:
I:2NO2(g)+NaCl(s)![]() NaNO3(s)+ClNO(g) K1
NaNO3(s)+ClNO(g) K1
II:2NO(g)+Cl2(g)![]() 2ClNO(g) K2
2ClNO(g) K2
��4NO(g)+2NaCl(s) ![]() 2NaNO3(s)+2NO(g)+Cl2(g)��ƽ�ⳣ��K=_____(��K1��K2��ʾ)��
2NaNO3(s)+2NO(g)+Cl2(g)��ƽ�ⳣ��K=_____(��K1��K2��ʾ)��
���ں��������£���2L�����ܱ������м���0.2molNO��0.1molCl2,10minʱ��ӦII�ﵽƽ�⡣���10min��v(ClNO)=7.5��10-3mol/(L��min),��ƽ��ʱNO��ת������1=_____�������������䣬��ӦII�ں�ѹ�����½��У�ƽ��ʱNO��ת������2___��1 (����>����<������=��)��