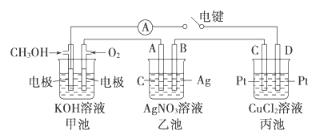
��Ŀ����
����Ŀ��̼̼˫����������ʾ�Ķ��ѷ�ʽ��
 ��
��
 ��
��
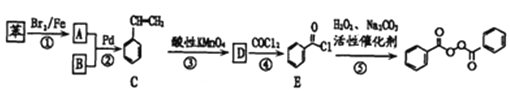
�߷��ӵ���A(C6H10O3)�ɽ������·�Ӧ����Ӧ��ͼ��

��֪��
��.�Կ�ͼ��ijЩ���������ʵ�˵����A�������²���NaHCO3��Һ��Ӧ��������Na��Ӧ�ų�H2��B����NaHCO3��Һ��Ӧ�ų�CO2��C����Na��Ӧ�ų�H2��D���ܣ�G�������¼Ȳ���NaHCO3��Һ��Ӧ��Ҳ����Na��Ӧ�ų�H2��
��.����һOH����ͬһ��Cԭ���ϵĽṹ���ȶ���
��1��д����Ӧ����(1)�ķ�Ӧ���ͣ�___________��д��D�ļ���ʽ��____________________��
��2��д������E���������ŵ����ƣ� ____________________��
��3��B��ŨH2SO4��������״����ȷ�Ӧ���ɵ��л����ϵͳ����Ϊ____________________��
��4��д��F��G�Ļ�ѧ��Ӧ����ʽ��____________________________________��
��5����B��Ϊͬ���칹�壬����Ϊ��״���������ʹ���______________�֣������������칹����
��6������ƺ���������������E�Ʊ��Ҷ���(����ԭ����ѡ���÷�Ӧ����ͼ��ʾ����ע����Ҫ�ķ�Ӧ����) ______________�����磺
![]()
���𰸡� ˮ��(��ȡ��)��Ӧ ![]() �ʻ����Ȼ� ����ϩ�����
�ʻ����Ȼ� ����ϩ����� 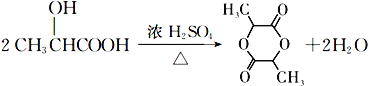 5
5 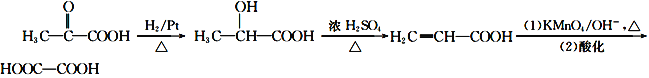
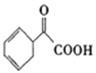
�����������������I��������A����NaHCO3��Һ��Ӧ��������Na��Ӧ�ų�H2��˵��A�в����Ȼ��������ǻ��������з�Ӧ��������Ӧ�IJ����֪��A�ܷ���ˮ�ⷴӦ����A�к���������B����NaHCO3��Һ��Ӧ�ų�CO2��˵��B�к����Ȼ���C����Na���÷ų�H2��D���ܣ�˵��C�Ǵ���C�������Ӽ���ˮ����D�����ݷ���ʽ֪��C���Ҷ�����D�Ľṹ��ʽΪ![]() ��G�������¼Ȳ���NaHCO3��Һ��Ӧ��Ҳ����Na���÷ų�H2��˵��G�в����Ȼ����ǻ�����ֻ��������F�к��д��ǻ����Ȼ���E�ܷ����ӳɷ�Ӧ˵��E�к��в����ͼ�������E�ķ���ʽ��������Ϣ֪��E�Ľṹ��ʽΪCH3COCOOH ����B�Ľṹ��ʽΪCH2=C(CH3)COOH��A�Ľṹ��ʽΪ��
��G�������¼Ȳ���NaHCO3��Һ��Ӧ��Ҳ����Na���÷ų�H2��˵��G�в����Ȼ����ǻ�����ֻ��������F�к��д��ǻ����Ȼ���E�ܷ����ӳɷ�Ӧ˵��E�к��в����ͼ�������E�ķ���ʽ��������Ϣ֪��E�Ľṹ��ʽΪCH3COCOOH ����B�Ľṹ��ʽΪCH2=C(CH3)COOH��A�Ľṹ��ʽΪ��![]() ��
��
��1����Ӧ����(1)�ķ�Ӧ������ˮ��(��ȡ��)��Ӧ��D�ļ���ʽΪ![]() ��
��
��2������E�������������ʻ����Ȼ���
��3��B��CH2=C(CH3)COOH����ŨH2SO4��������״����ȷ�Ӧ���ɵ��л����ϵͳ����Ϊ����ϩ�������
��4��F��G�Ļ�ѧ��Ӧ����ʽΪ ��
��
��5����B��CH2=C(CH3)COOH����Ϊͬ���칹�壬����Ϊ��״������������CH2=CH COOCH3��HCOOCH=CHCH3��HCOOCH2CH=CH2��CH3COOCH=CH2��HCOOC(CH3)=CH2������5�֡�
��6��������E�Ʊ��Ҷ�������������E�������ӳ�����2-�ǻ����ᣬȻ��2-�ǻ����ᷢ����ȥ��Ӧ�õ���ϩ�ᣬ����ϩ�ᾭ����������������ữ�õ��Ҷ��ᡣ����ϳ�·�����£�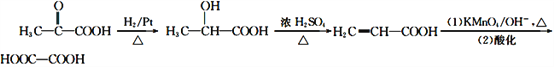 ��
��

 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д�����Ŀ�����ᾧ�����ɿɱ�ʾΪH2C2O4��xH2O��ij�о���ѧϰС������ͼװ�ý��������ᾧ�����ȷֽ�IJ��ֲ������֤����ʵ�顣��ش��������⡣

�����ϲ��ġ�
�����ᾧ����101 ��ʱ��ʼ�ۻ���150 ��ʱ��ʼ������175 ��ʱ��ʼ�ֽ⣻
������ƺͲ�����ƾ�Ϊ��ɫ�����
��1��������ͼ��ʾ��װ����ͨ��ʵ�������ᾧ��IJ��ַֽ������װ��B�пɹ۲쵽������ð���ҳ���ʯ��ˮ��������ɴ˼�ͬѧ�жϲ��ᾧ��ֽ�IJ�������CO2�����������ͬѧ�������䷴�Ե����ɿ�����______________________________________��
��2����ͬѧ��Ϊ���ᾧ��ֽ�IJ����к���CO��Ϊ������֤��XӦѡ��________(�ѧʽ)Ũ��Һ��װ��D��������____________________��
��3��ʵ��������漰���²������ٵ�ȼװ��A���ľƾ��ƣ���Ϩ��װ��A���ľƾ��ƣ��۵�ȼװ��E���ľƾ��ƣ���Ϩ��װ��E���ľƾ��ơ���4���������ȵ����˳��Ϊ____________(�����)����ȼE���ƾ���ǰ����Ҫ���еIJ�����______________��
��4��ʵ������з���װ��E�к�ɫ��ĩ���ɫ��װ��F���к�ɫ���������������װ��F�еĹ���Ϊ������������װ��F�з�����Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________��
��5����ͬѧ�õζ����ⶨ���ᾧ���нᾧˮ�ĺ��������������в�����
����һ���÷�����ƽ��ȡ3.15 g�����ĸò��ᾧ�������Ƴ�250 mL��Һ��
�����������Һ����ȡ25.00 mL���������Һ����ƿ�������������������ữ��
��������ȡ0.100 mol��L��1������KMnO4��Һ�����еζ������ν�����±���ʾ��
��һ�� | �ڶ��� | ������ | |
������Һ���(mL) | 25.00 | 25.00 | 25.00 |
����Һ���(mL) | 9.99 | 10.01 | 10.00 |
��֪�ζ���Ӧ�����ӷ���ʽΪ��MnO![]() ��H2C2O4��H���D��Mn2����CO2����H2O(δ��ƽ)��
��H2C2O4��H���D��Mn2����CO2����H2O(δ��ƽ)��
�����Ʋ�����Һ�IJ������������ǣ������������ձ�������ˮ�ܽ�������Һת����________��ϴ�ӣ����ݣ�ҡ�ȡ�
��ͨ������ȷ��x��________��
����Ŀ������ֻʢ����ͬ��NaOH��Һ���ձ���ͨ�벻ͬ����CO2���壬��������Һ����μ���ϡ������������������Һ���ȣ�������CO2������HCl���ʵ����Ĺ�ϵ��ͼ��������CO2���ܽ��HCl�Ļӷ����������з�������ȷ�������

��Ӧͼ�� | ��Һ�е���Ҫ�ɷ� | |
A | �� | NaOH��NaHCO3 |
B | �� | NaHCO3��Na2CO3 |
C | �� | NaOH��Na2CO3 |
D | �� | Na2CO3 |
A. A. B. B C. C D. D