��Ŀ����
����Ŀ��2SO2(g)+O2(g)��2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2(g)����Ϊ1mol SO3�� ��H=��99kJ��mol-1����ش��������⣺
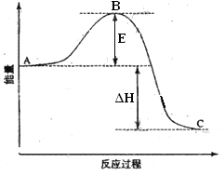
��1��ͼ��A��C�ֱ��ʾ________��_________��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿_______���÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ�_________��������_________________________��
��2��ͼ����H=____________kJ��mol-1��
��3�������Ӧ�����ԣ�SO2��Ϊ0.05 mol��L-1��min-1�����ԣ�O2��=_________��(SO3)=___________��
��4����֪�������ȼ����Ϊ296 kJ��mol-1��������S(s)����3 molSO3(g)����H��Ҫ��д��������̣�_______��
���𰸡���Ӧ�������� ������������ ��Ӱ�� ���� �����ı䷴Ӧ����,���ͻ�� E��198 0.025 mol��L-1��min-1 0.05 mol��L-1��min-1 ��H=��1185kJ/mol
��������
��1������ͼʾ��ͼ��A��ʾ��Ӧ��������C��ʾ������������E��ʾ��Ӧ�Ļ�ܣ���Ӧ��ֻ�뷴Ӧ����������������������Դ�С�йأ������أ�E�Ĵ�С�Է�Ӧ����Ӱ�졣V2O5���������ı䷴Ӧ���̣����ͷ�Ӧ�Ļ��E���Ӷ�ʹͼ��B�㽵�͡�
��2��1 mol SO2��g������Ϊ1 mol SO3��g������H����99 kJ/mol����Ӧ2SO2��g����O2��g��![]() 2SO3��g����H=��198kJ/mol����ͼ����H=��198kJ/mol��
2SO3��g����H=��198kJ/mol����ͼ����H=��198kJ/mol��
��3������ͬһ��Ӧ�в�ͬ���ʱ�ʾ�Ļ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȿ�֪������O2��=0.5����SO2��=0.025mol/��L��min��������SO3��=����SO2��=0.05mol/��L��min����
��4���������ȼ����Ϊ296 kJ/mol�����ʾSȼ���ȵ��Ȼ�ѧ����ʽΪ�٣�S��s��+O2��g��=SO2��g����H=��296kJ/mol������Ϊ�ڣ�2SO2��g����O2��g��![]() 2SO3��g����H=��198kJ/mol��Ӧ�ø�˹���ɿ�֪��+�ڡ�0.5�ã�S��s��+3/2O2��g��=SO3��g����H=����296kJ/mol��+0.5������198kJ/mol��=��395kJ/mol���������3molSO3��g������H=����395kJ/mol����3=��1185kJ/mol��
2SO3��g����H=��198kJ/mol��Ӧ�ø�˹���ɿ�֪��+�ڡ�0.5�ã�S��s��+3/2O2��g��=SO3��g����H=����296kJ/mol��+0.5������198kJ/mol��=��395kJ/mol���������3molSO3��g������H=����395kJ/mol����3=��1185kJ/mol��

����Ŀ���±����Ǹ��鷴Ӧ�ķ�Ӧ��ͷ�Ӧ�¶ȣ���Ӧ�տ�ʼʱ���ų�H2���������ǣ�������
��� | ��������ĩ״�� | ���ʵ�����mol�� | ���Ũ�ȼ���� | ��Ӧ�¶ȣ��棩 |
A | Al | 0.1 | 2mol.L-1����10mL | 60 |
B | Fe | 0.1 | 1mol.L-1����10mL | 50 |
C | Mg | 0.1 | 1mol.L-1����10mL | 60 |
D | Mg | 0.1 | 1mol.L-1����10mL | 60 |
A.AB.BC.CD.D