��Ŀ����
��֪��һ��ɫ��ĩ����NaCl��Na2SO4��Na2CO3��CuSO4��MgCl2�е�һ�ֻ�����ɡ�ijͬѧ��̽����һ��ĩ����ɣ���������ʵ�飺?
��ȡ������ĩ����ˮ�ܽ⣬����ɫ����Һ��?
��ȡ������Һ����������NaOH��Һ����������������?
����ȡ������ĩ������ϡ���ᣬ��������������?
(1)������ݸ�ͬѧʵ�����������Ʋ���һ��ĩ�Ŀ��������
________________________________��?
(2)���Ʋ�÷�ĩֻ��Na2SO4��ɣ������ڸ�ͬѧʵ���������ƺ���ʵ�飬��ȷ�������ĩ����ɡ����ɹ�ѡ����Լ��У�BaCl2��Һ��AgNO3��Һ��ϡHNO3��NaOH��Һ��Na2CO3��Һ��Ba(NO3)2��Һ��ϡ���ᡳ
ע��ʵ�鲽����������˳���š�ʵ���������ӷ���ʽ�ͽ���Ҫ����Ӧ��ʵ�鲽��һһ��Ӧ��?????????
��ȡ������ĩ����ˮ�ܽ⣬����ɫ����Һ��?
��ȡ������Һ����������NaOH��Һ����������������?
����ȡ������ĩ������ϡ���ᣬ��������������?
(1)������ݸ�ͬѧʵ�����������Ʋ���һ��ĩ�Ŀ��������
________________________________��?
(2)���Ʋ�÷�ĩֻ��Na2SO4��ɣ������ڸ�ͬѧʵ���������ƺ���ʵ�飬��ȷ�������ĩ����ɡ����ɹ�ѡ����Լ��У�BaCl2��Һ��AgNO3��Һ��ϡHNO3��NaOH��Һ��Na2CO3��Һ��Ba(NO3)2��Һ��ϡ���ᡳ
| ʵ�鲽�� | ʵ������ | ��Ӧ���ӷ���ʽ�ͽ��� |
| | | |
(1)ֻ��NaCl��ֻ��Na2SO4����NaCl��Na2SO4��ɣ�?
(2)
(2)
| ʵ�鲽�� | ʵ������ | ��Ӧ���ӷ���ʽ�ͽ��� |
| ��ȡ������Һ�������μ������ữ��Ba(NO3)2��Һ������? ��ȡ�ٵ���Һ�ټ��������ữ��AgNO3��Һ | ������ɫ���� ���������� | Ba2++SO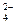 ====BaSO4�� ====BaSO4��˵��Na2SO4���� ˵����Һ����Cl- ���Ը÷�ĩ���ֻ��Na2SO4 |
��ʵ��ٿ�֪�ð�ɫ��ĩ�в�������ͭ���ɢڿ�֪�ð�ɫ��ĩ�в����Ȼ�þ���ɢۿ�֪���в���̼���ƣ����Ըð�ɫ�������ɿ��������¼����������ֻ���Ȼ��ƣ���ֻ�������ƣ������Ȼ��ƺ���������ɡ�������������ӵĴ��ڿ���ѡ�ñ��Ρ�?

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 2Na�����������������
2Na����������������� ������
������