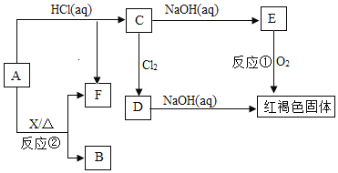
��Ŀ����
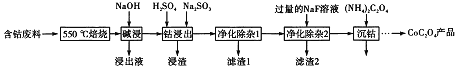
����Ŀ����������;�㷺��������ָʾ���ʹ����Ʊ���һ������ˮ�ܿ����Ҫ�ɷ�ΪCo2O3��������Fe2O3��Al2O3��MnO��MgO��CaO�ȣ���ȡCoC2O4�����������£�

��֪��
�ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Ca2+��Mg2+��Al3+�ȣ�
������������Co3+������������ǿ��
������������������������ʽ��ȫ����ʱ��Һ��pH���±���
������ | Fe(OH)3 | Fe(OH)2 | Co(OH)2 | Al(OH)3 | Mn(OH)2 |
��ȫ������pH | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
��1�����������м���Na2SO3��Ŀ���ǽ���ԭ_________�������ӷ��ţ���
��2������ƽ���ƶ�ԭ��������Na2CO3��ʹ����Һ��Fe3+��Al3+ת�����������������ԭ���ǣ�___________________________________________��
��3�������ơ�þ���ǽ���Һ��Ca2+��Mg2+ת��ΪMgF2��CaF2���������������NaF��������Һc(Mg2+)/ c (Ca2+)=______________________���� ��֪��Ksp(MgF2)=7.35��10-11��Ksp(CaF2)=1.00��10-10��
��4����֪��NH3��H2O![]()
![]() +OH Kb=1.8��105��
+OH Kb=1.8��105��
H2C2O4![]() H++
H++![]() Ka1=5.4��102��
Ka1=5.4��102��
![]() H++
H++![]() Ka2=5.4��105��
Ka2=5.4��105��
����(NH4)2C2O4��Һ������Ũ���ɴ�С��˳��Ϊ______________________��
��5��
���𰸡� Fe3+��Co3+ ��Һ�д���ƽ��Fe3����3H2O![]() Fe(OH)3��3H����Al3����3H2O
Fe(OH)3��3H����Al3����3H2O![]() Al(OH)3��3H��������̼���ƺ���H����CO32-��Ӧ��ʹˮ��ƽ���������Ӷ��������� 0.735 c(
Al(OH)3��3H��������̼���ƺ���H����CO32-��Ӧ��ʹˮ��ƽ���������Ӷ��������� 0.735 c(![]() )��c(
)��c(![]() )��c(H+)��c(
)��c(H+)��c(![]() )��c(OH) ��ȴ�ᾧ
)��c(OH) ��ȴ�ᾧ
�����������黯ѧ�������̣���1������ˮ�ܿ�ijɷ֣���Fe3����Co3������ǿ�����ԣ���˼���Na2SO3Ŀ�Ļ�ԭCo3����Fe3������2����Һ�д���ƽ��Fe3����3H2O![]() Fe(OH)3��3H����Al3����3H2O
Fe(OH)3��3H����Al3����3H2O![]() Al(OH)3��3H��������̼���ƺ�H����CO32-��Ӧ��ʹˮ��ƽ�����ƣ��Ӷ�������������3��
Al(OH)3��3H��������̼���ƺ�H����CO32-��Ӧ��ʹˮ��ƽ�����ƣ��Ӷ�������������3��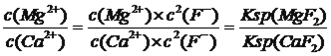 =7.35��10��11/1.00��10��10=0.735����4������ƽ�ⳣ��Ka1>Ka2>Kb��˵��NH4����ˮ��̶ȴ���C2O42����ˮ��̶ȣ���ҺӦ�����ԣ�����ˮ��̶��������ݻ�ѧʽ��c(NH4��)��࣬�������Ũ�ȴ�С˳����c(NH4��)>c(C2O42��)>c(H��)>c(HC2O4��)>c(OH��)����5����ȡCoC2O4��2H2O����Ҫ���еIJ���ʱ����Ũ������ȴ�ᾧ�����ˡ�
=7.35��10��11/1.00��10��10=0.735����4������ƽ�ⳣ��Ka1>Ka2>Kb��˵��NH4����ˮ��̶ȴ���C2O42����ˮ��̶ȣ���ҺӦ�����ԣ�����ˮ��̶��������ݻ�ѧʽ��c(NH4��)��࣬�������Ũ�ȴ�С˳����c(NH4��)>c(C2O42��)>c(H��)>c(HC2O4��)>c(OH��)����5����ȡCoC2O4��2H2O����Ҫ���еIJ���ʱ����Ũ������ȴ�ᾧ�����ˡ�