��Ŀ����
������������:��ͭ�����Ǣ�CuO��NaHSO4 ��Ba��OH��2�������������ɱ��ᵨ����ˮ
��1���������ʷ����в����ڵ���ʵ��� ������ţ�������ˮ��Һ�еĵ��뷽��ʽΪ ��
��2��̼��������������մ����������ʳ�����ơ���һ�������£���������������������̼������ʳ�ε������� ������ţ���
��3������������������������ˮ��Һ�з�����Ӧ,�����ӷ���ʽΪ:H++OH-=H2O,��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4����� ��Һ�л����μӢ� ��Һ�������Һ�պó�����ʱ�����ӷ���ʽΪ��_______��
��5������������������������һ������������ÿ�����һ�������ʡ���ʽ̼��ͭ��Cu2��OH��2 CO3]����д���÷�Ӧ�Ļ�ѧ��Ӧ����ʽ________________ ��
��1�� �٢ڢޢߢ� NaHSO4=Na++H++SO42-
��2���ܢݢߢ��
��3��Ba��OH��2+2HCl=BaCl2+2H2O
��4��2OH-+SO42-+Ba2++2H��=2H2O+BaSO4��
��5��2Cu+O2+CO2+H2O=Cu2��OH��2 CO3
���������������1���������ָ��ˮ��Һ�������״̬�µ���Ļ���������ʵķ���Ƕ����������ᡢ��Ρ����������ˮ�ȣ�����ֻ�Тۢܢݢ��ʱ����ʣ�NaHSO4����ʽ���Σ�����������ǿ�ᣬ������ˮ��Һ�еĵ��뷽��ʽΪ��NaHSO4=Na++H++SO42-��
��2������̼������ʳ��ʵ��������Cl-��CO32-����CO32-��Ӧ�������Тܢݢߢ�ᣬ�ܢ߲������壬�ݢ������������ᱻ���ա�
��3��H++OH-=H2O�����ӷ���ʽ��ʾǿ����ǿ�����������εķ�Ӧ��������10��������ֻ�Тݢ߷��ϣ��仯ѧ����ʽΪ��Ba��OH��2+2HCl=BaCl2+2H2O��
��4����Һǡ�ó�����ʱ˵��H+��OH-ǡ����ȫ��Ӧ�����������������H+��OH-�ĸ����ȣ��ɵ� 2 OH-+SO42-+Ba2++2H��=2H2O+BaSO4��
��5��������ĿҪ��������������һ������������ÿ�����һ�������ʡ���ʽ̼��ͭ��Cu2��OH��2 CO3]�����������ʵĻ�ѧʽ���ѵó����������ʷֱ���ͭ��������ˮ��������̼��
���㣺�������ʵ��жϡ����Ӽ������ӷ���ʽ�����塢���ӷ���ʽ����д����ѧʽ�ķ���

 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�������A��B����ѧ���������ʣ����������ӿɴ��±���ѡ��
| ������ | K����Na����Fe2����Ba2����NH4+ |
| ������ | OH����NO3����I����HCO3����AlO2����HSO4�� |
(1)��A��B��ˮ��Һ��Ϊ��ɫ��B��ˮ��Һ�ʼ��ԣ��һ�Ϻ�ֻ����������ϡ����İ�ɫ��������ʹ��ɫʯ����ֽ���������塣
��B�Ļ�ѧʽΪ__________________��
��A��B��Һ��Ϻ���ȳ����ԣ���Ӧ�����ӷ���ʽΪ__________________________��
(2)��A��ˮ��Һ��dz��ɫ��B��ˮ��Һ��ɫ������ɫ��ӦΪ��ɫ����A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ���A��B��ˮ��Һ����������Ա仯����
��AΪ_______________��
�ھ�����������������Һ��Ƶ�ԭ����������֣�
��._______ _____________________�� ��.___________ ____________��
������һ������֤��������Һ��Ƶ�ԭ��__________________________________��
��������Һ���ԭ����������Ƴ�ԭ��أ���������a����b����b���ĵ缫��ӦʽΪ_______________________________________
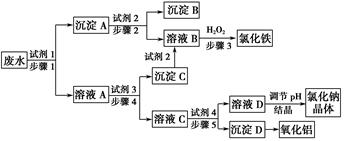
 ��
��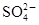 ��Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺
��Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺