��Ŀ����
ijУ��ѧ��ȤС��ѧ��ȡ������Na2SO3?7H2O 50.00g��Ȼ�����������600�����ϵ�ǿ�������أ���ȫ��Ӧ����������������������غ�Ĺ��������൱����ˮ�������Ƶļ���ֵ�����Ҹ�Ԫ�ص����Ҳ����ˮ�������Ʒ��ϣ�����������ˮ��ȴ������Һ�ļ��Դ���������ֵ��������ϸ˼������λͬѧ����Na2SO3���Ⱥ������绯��Ӧ�Ӷ��ܺý������ַ��������������һ��ʵ�飬��֤���Լ��Ľ�������ȷ�ģ�
��1������Na2SO3?7H2OʱΪʲôҪ�������������ü�Ҫ���ֺͻ�ѧ����ʽ��ʾ����______��
��2�����Է�Ӧ����Ľ�����______���û�ѧ����ʽ���
��3����Һ�ļ��Դ���������ֵ��ԭ���ǣ��������ӷ���ʽ���______��
��4��������������ʲôʵ������֤���IJ��룮����д��һ�ֿ��е�ʵ�鷽������
| ʵ���Լ� | ���� | ���� | ���� | �йط���ʽ |
| ______ | ______ | ______ | ______ | ______ |
�⣺��1�����������ڿ������ױ���������Ϊ�����ƣ���Na2SO3?7H2OʱҪ������������Ӧ�Ļ�ѧ����ʽΪ��2Na2SO3+O2=2Na2SO4��
�ʴ�Ϊ��Na2SO3�ڿ����м���ʱ�ױ���������ΪNa2SO4��2Na2SO3+O2=2Na2SO4��
��2��Na2SO3���Ⱥ������绯��Ӧ����Ԫ�ػ��ϼ۴�+4�۱仯Ϊ-2�ۺ�+6�ۣ���Ӧ�Ļ�ѧ����ʽΪ��4Na2SO3=Na2S+3Na2SO4��
�ʴ�Ϊ��4Na2SO3=Na2S+3Na2SO4��
��3����Һ�ļ��Դ���������ֵ��ԭ���ǣ�Na2SO3���Ⱥ������绯��Ӧ���������ƣ���Һ��������ˮ��̶ȴ��������������ˮ��̶ȣ���Һ������ǿ����Ӧ�����ӷ���ʽΪ��S2-+H2O?HS-+OH-���ʴ�Ϊ��S2-+H2O?HS-+OH-��
��4���������ܺ��ᷴӦ�������⣬�����ӵļ��鷽����ȡ�������Ⱥ�Ĺ������������ˮ�����Һ��ȡ��Һ�������Թ��У������м�ϡ���ᣬ���г���������ζ�������ɣ���֤����S2-����������ӿ��Ժͱ����ӷ�Ӧ�������ᱵ���ټ����Ȼ�����Һ���ְ�ɫ����֤��������������ӣ����ӷ���ʽΪS2-+2H+=H2S����Ba2++SO42-=BaSO4����
�ʴ�Ϊ��
��
��������1�����������ڿ������ױ���������Ϊ�����ƣ�
��2��Na2SO3���Ⱥ������绯��Ӧ����Ԫ�ػ��ϼ۴�+4�۱仯Ϊ-2�ۺ�+6�ۣ�
��3��Na2SO3���Ⱥ������绯��Ӧ���������ƣ���Һ��ˮ��̶ȴ����������Ƶ�ˮ��̶ȣ���Һ��С��ǿ��
��4������Na2SO3���Ⱥ������绯��Ӧ�������ƺ������ƣ����ʵ����Լ���S2-��SO42-�ķ��������ԣ�
���������⿼�����������Ƶ����ʷ����жϣ���Ӧ��ѧ����ʽ�����ӷ���ʽ����д������ʵ�鷽������ƺ�ʵ��������ж��ǽ���ؼ�����Ŀ�Ѷ��еȣ�
�ʴ�Ϊ��Na2SO3�ڿ����м���ʱ�ױ���������ΪNa2SO4��2Na2SO3+O2=2Na2SO4��
��2��Na2SO3���Ⱥ������绯��Ӧ����Ԫ�ػ��ϼ۴�+4�۱仯Ϊ-2�ۺ�+6�ۣ���Ӧ�Ļ�ѧ����ʽΪ��4Na2SO3=Na2S+3Na2SO4��
�ʴ�Ϊ��4Na2SO3=Na2S+3Na2SO4��
��3����Һ�ļ��Դ���������ֵ��ԭ���ǣ�Na2SO3���Ⱥ������绯��Ӧ���������ƣ���Һ��������ˮ��̶ȴ��������������ˮ��̶ȣ���Һ������ǿ����Ӧ�����ӷ���ʽΪ��S2-+H2O?HS-+OH-���ʴ�Ϊ��S2-+H2O?HS-+OH-��
��4���������ܺ��ᷴӦ�������⣬�����ӵļ��鷽����ȡ�������Ⱥ�Ĺ������������ˮ�����Һ��ȡ��Һ�������Թ��У������м�ϡ���ᣬ���г���������ζ�������ɣ���֤����S2-����������ӿ��Ժͱ����ӷ�Ӧ�������ᱵ���ټ����Ȼ�����Һ���ְ�ɫ����֤��������������ӣ����ӷ���ʽΪS2-+2H+=H2S����Ba2++SO42-=BaSO4����
�ʴ�Ϊ��
| ʵ���Լ� | ���� | ���� | ���� | �йط���ʽ |
| �Ȼ�����Һ������ | ȡ�������Ⱥ�Ĺ������������ˮ�����Һ��ȡ��Һ�������Թ��У������м�ϡ���������Һ�м����Ȼ�����Һ | �г���������ζ�����������ɣ��������ᱵ��ɫ�� | ֤���ֽ�����к������ƺ������� | S2-+2H+=H2S����Ba2++SO42-=BaSO4�� |
��������1�����������ڿ������ױ���������Ϊ�����ƣ�
��2��Na2SO3���Ⱥ������绯��Ӧ����Ԫ�ػ��ϼ۴�+4�۱仯Ϊ-2�ۺ�+6�ۣ�
��3��Na2SO3���Ⱥ������绯��Ӧ���������ƣ���Һ��ˮ��̶ȴ����������Ƶ�ˮ��̶ȣ���Һ��С��ǿ��
��4������Na2SO3���Ⱥ������绯��Ӧ�������ƺ������ƣ����ʵ����Լ���S2-��SO42-�ķ��������ԣ�
���������⿼�����������Ƶ����ʷ����жϣ���Ӧ��ѧ����ʽ�����ӷ���ʽ����д������ʵ�鷽������ƺ�ʵ��������ж��ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ
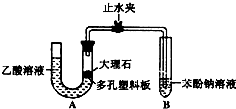 ijУ��ѧ��ȤС��ѧ��Ϊ��̽�����ᡢ̼��ͱ��ӵ�����ǿ������Уѧ������װ����ͼ��ʵ��װ�ã��г���������ȥ��������������һЩʵ�飺
ijУ��ѧ��ȤС��ѧ��Ϊ��̽�����ᡢ̼��ͱ��ӵ�����ǿ������Уѧ������װ����ͼ��ʵ��װ�ã��г���������ȥ��������������һЩʵ�飺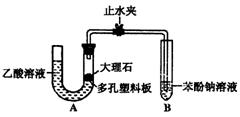 ijУ��ѧ��ȤС��ѧ��Ϊ��̽�����ᡢ̼��ͱ��ӵ�����ǿ������Уѧ������װ����ͼ��ʵ��װ�ã��г���������ȥ����������
ijУ��ѧ��ȤС��ѧ��Ϊ��̽�����ᡢ̼��ͱ��ӵ�����ǿ������Уѧ������װ����ͼ��ʵ��װ�ã��г���������ȥ����������