��Ŀ����
14�� H��C��N��F��Al��Ca��Ni��Cu����ѧ�γ�����Ԫ�أ��ش����漸��С��
H��C��N��F��Al��Ca��Ni��Cu����ѧ�γ�����Ԫ�أ��ش����漸��С����1����̬Ni����Χ�����Ų�ʽΪ3d84s2
��2���Ƚ�C��N��Al�ĵ�һ������N��C��Al����Ԫ�ط��ű�ʾ��
��3���Ƚ�Cu��Al��C�����������ķе��ɸߵ���˳��ΪAl2O3��CuO��CO2���û�ѧʽ��ʾ��
��4��������ij��̬�л�����C��O��H����Ԫ����ɣ�д������C���ӻ���ʽsp2�ӻ�
��5��NiΪ����г����IJ��ϣ���Al��NiOOHΪ�缫��NaOHΪ���Һ��ɵ�أ��ŵ�ʱNiOOHת��ΪNi��OH��2д����ص��ܷ�ӦAl+3NiOOH+NaOH+H2O=3Ni��OH��2+NaAlO2
��6��Ca��F�γɵ�ij���Ӿ���ṹ��ͼ��ʾ��Ca2+����λ��Ϊ8��
���� ��1��Ni��28��Ԫ�أ���Χ�����Ų�ʽΪ3d84s2��
��2��ͬ����Ԫ�ش�����Ԫ�صĵ�һ��������������A����A����A����A��
��3��Cu��Al��C�����������ֱ�Ϊ����ͭ����������������̼��������̼Ϊ���Ӿ��壻����ͭ���������ɽ��Ӱ�����Ӿ���������жϣ�
��4��ij��̬�л�����C��O��H����Ԫ����ɣ�ӦΪ��ȩ��
��5��NiOOHת��ΪNi��OH��2��MiԪ�ػ��ϼ۽��ͣ�����ԭ��Ϊ������Ӧ������������Al����������NaAlO2��
��6��Ca��F�γɵ����ӻ�����ΪCaF2���ɾ�����֪Ca2+λ�ڶ��㣬ÿ����������1��F-��Ca2+���������
��� �⣺��1��Ni��28��Ԫ�أ���Χ�����Ų�ʽΪ3d84s2���ʴ�Ϊ��3d84s2��
��2��ͬ����Ԫ�ش�����Ԫ�صĵ�һ��������������N��C��Al���ʴ�Ϊ��N��C��Al��
��3��Cu��Al��C�����������ֱ�Ϊ����ͭ����������������̼��������̼Ϊ���Ӿ��壬����ͭ����������Ϊ���Ӿ��壬�������������Ӱ뾶��С����ɽ϶࣬�������������ܽϴе�ϸߣ�ӦΪAl2O3��CuO��CO2��
�ʴ�Ϊ��Al2O3��CuO��CO2��
��4����̬�л�����C��O��H����Ԫ����ɣ�ΪHCHO��C�γ�3���ļ���û�й¶Ե��ӣ�Ϊsp2�ӻ����ʴ�Ϊ��sp2�ӻ���
��5��NiOOHת��ΪNi��OH��2��MiԪ�ػ��ϼ۽��ͣ�����ԭ��Ϊ������Ӧ������������Al����������NaAlO2����Ӧ�ķ���ʽΪAl+3NiOOH+NaOH+H2O=3Ni��OH��2+NaAlO2��
�ʴ�Ϊ��Al+3NiOOH+NaOH+H2O=3Ni��OH��2+NaAlO2��
��6��Ca��F�γɵ����ӻ�����ΪCaF2���ɾ�����֪Ca2+λ�ڶ��㣬ÿ����������1��F-��Ca2+���������������Ϊ8���������У�����λ��Ϊ8���ʴ�Ϊ��8��
���� ���⿼���Ϊ�ۺϣ��漰�����������Ų�������е�Ƚ��Լ��ӻ����͵��жϣ�Ϊѡ�����������Ҫ�����Լ��߿��������ͣ�������˫���Ŀ��飬ע����ػ���֪ʶ�Ļ��ۣ��ѶȲ���


| A�� | X��ij�ֵ�����һ����������ˮ������ | |
| B�� | �����Ӱ뾶��W-��R2-��X2-��S3+ | |
| C�� | R��Z�������γ����ӻ����� | |
| D�� | ��ĸ��������Ԫ��������������ˮ����������ǿ����Y |
| A�� | 0.1 mol•L-1CH3COONa��Һ��0.1 mol•L-1HCl��Һ�������ϣ�c��Na+��=c��Cl-����c��CH3COO-����c��OH-�� | |
| B�� | 0.1 mol•L-1NH4Cl��Һ��0.1 mol•L-1��ˮ�������ϣ�pH��7����c��NH3•H2O����c��NH4+����c��Cl-����c��OH-�� | |
| C�� | 0.1 mol•L-1Na2CO3��Һ��0.1 mol•L-1NaHCO3��Һ�������ϣ�$\frac{2}{3}$c��Na+��=c��CO32-��+c��HCO3-��+c��H2CO3�� | |
| D�� | 0.1 mol•L-1Na2C2O4��Һ��0.1 mol•L-1HCl��Һ�������ϣ�H2C2O4Ϊ��Ԫ���ᣩ��2c��C2O42-��+c��HC2O4-��+c��OH-��+c��Cl-��=c��Na+��+c��H+���� |
��1��Sλ��Ԫ�����ڱ������ڵ�VIA�壬Fe�Ļ�̬ԭ�Ӻ���۵����Ų�ʽΪ3d64s2����1s22s22p63s23p63d64s2����O�Ļ�̬ԭ�Ӻ�����8���˶�״̬��ͬ�ĵ���
��2���������������=�����
| ��һ������ | ������ | �е� | �ǽ����� |
| N��S | Fe3+��Al3+ | NH3��H2O | 16O=18O |
4Fe��s��+302��g��=2Fe2O3��s����H=-aKJ/mol
4Al��s��+3O2��g��=2Al2O3��S����H=-bKJ/mol
�tAl��s���ĵ��ʺ�Fe2O3��s����Ӧ���Ȼ�ѧ����ʽ��Fe2O3��s��+2Al��s��=Al2O3��s��+2Fe��s����H=-$\frac{1}{2}$��b-a��KJ•mol-1
��4��FeSO4��Һ�������ᷢ����Ӧ��д���˷�Ӧ�����ӷ���ʽ�����������ת�Ƶķ������Ŀ
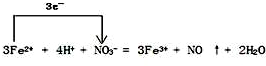 ��
�� | A�� | ��ʽ�е�XΪS4O62- | |
| B�� | �÷�Ӧ�еĻ�ԭ��ΪFe2+��S2O32- | |
| C�� | ��3 mol Fe2+������ʱ����Fe2+��ԭ��O2�����ʵ���Ϊ1 mol | |
| D�� | ������1 mol Fe3O4ʱ��ת�Ƶ��ӵ����ʵ���Ϊ4 mol |

| A�� | ���л������ڱ���ͬϵ�� | |
| B�� | 1mol���л�����ȫȼ��������25molO2 | |
| C�� | ���л�������к���21��̼ԭ�� | |
| D�� | ���л����һ�ȴ���ֻ��6�� |
| A�� | ͬ��ͬѹͬ�����CO2��SO2������ԭ������Ϊ2NA | |
| B�� | 25��ʱ��pH=13��1.0L Ba��OH��2��Һ�к��е�OH-��ĿΪ0.1NA | |
| C�� | 0.10molFe��������ˮ������Ӧ���ɵ�H2������Ϊ0.10NA | |
| D�� | 1L1.0mol•L-1NH4Cl��2L 0.5mol•L-1NH4Cl��Һ��NH4+��Ŀ��ͬ |
| A�� | �Ҵ������еĹ������Ƿ��ǻ� | |
| B�� | ʹ��K2Cr2O7������Һ���Ƽݣ����������Ҵ��������� | |
| C�� | �Ҵ����Է���ȡ������ȥ��������Ӧ | |
| D�� | ��75%���Ҵ���98%��Ũ������ҺѸ�ټ�����170��C����ȡ��ϩ |
| A�� | ��10ml0.1mol•L-1Na2CO3��Һ��εμӵ�10ml0.1mol•L-1�����У�c��Na+����c��Cl-����c��CO32-����c��HCO3-�� | |
| B�� | ����CO2 ͨ��0.1 mol•L-1Na2CO3��Һ������Һ���ԣ�����Һ��2 c��CO32����+c��HCO3����=0.1 mol•L-1 | |
| C�� | �����£�����ͬ�����pH=3�����pH=11һԪ��BOH��Һ��ϣ�������Һ����Ϊ����Ҳ����Ϊ���� | |
| D�� | ����������ʵ���Ũ�ȵ�NaClO��aq����NaCl��aq��������������С��Nǰ��N�� |