��Ŀ����
����Ŀ��[��ѧ-ѡ��3�����ʽṹ������]
��������һ�ִ��Բ��ϣ����й㷺��Ӧ�á� -
(1)��̬��ԭ�ӵĺ�������Ų�ʽΪ[Ar]_______��
(2)��ҵ�Ʊ������峣ʹ��ˮ�ⷨ���Ʊ�ʱ����������[CO(NH2)2 ]�������Ƶȼ������ʡ����ط��������ֲ�ͬԪ�صĵ縺���ɴ���С��˳����____________����������̼ԭ�ӵ��ӻ�������_________��
(3)��ҵ�Ʊ�������Ҳ��ʹ�ó��������Ʊ�ʱ�����백��NH3)������(N2H4)������Ƚ��±��а�(NH3)������(N2H4)���۷е㣬������ߵ͵���Ҫԭ��________��
N2H4 | NH3 | |
�۵�/�� | 2 | -77.8 |
�е�/�� | 113.5 | -33.5 |
(4)��ͼ�Ǵ����������Ӿ���Fe3O4�У�ȡ�����������侧��ṹ��һ�������壬�����е��������Ƿ��������������ܶѻ�______(��ǡ��������������Dz���Fe3O4�ľ���______(��ǡ����������������������Ӵ���������Χ�ɵ�_____��϶(��ռ�ṹ����
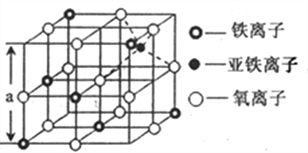
(5)����Fe3O4�����ܵ����ԭ��________��������ͼ����Fe3O4������ܶ�_____gcm-3�� (ͼ��a=0.42nm��������������λ��Ч���֣�
���𰸡� 3d64s2 O>N>C>H sp3�ӻ���sp2�ӻ� ��Ҫԭ�����������Ӽ��γɵ������Ŀ���ڰ����Ӽ��γɵ���� �� �� �������� ���ӿ������ֲ�ͬ��̬�������Ӽ���ٷ���ת�� 5.2g/cm3
��������(1)���ĺ����������Ϊ26�����̬��ԭ�ӵĺ�������Ų�ʽΪ[Ar]3d64s2 ��
(2)����[CO(NH2)2 ]��������Ԫ�طֱ�ΪN��H��C��O��Ԫ�صķǽ�����Խǿ���縺��Խ��������Ԫ�صĵ縺���ɴ���С��˳���ǣ�CH3COONa�м��е�Cԭ�� sp�ӻ����Ȼ��е�Cԭ�� sp�ӻ���
(3)�����������Ӽ��������������������Ӽ��γɵ������Ŀ���ڰ����Ӽ��γɵ�������������ķе����Ը��ڰ���
(4)��ͼʾ��֪�����е������ӹ��������������ܶѻ���������������������ĿΪ12��![]() +1=4��Fe3+����ĿΪ4��
+1=4��Fe3+����ĿΪ4��![]() +3��
+3��![]() =2��Fe2+����ĿΪ1����Fe��O��ԭ����ĿΪ3:4����Fe3O4�ľ�������������������������Χ��6��������Χ�ɵ����������϶���ġ�
=2��Fe2+����ĿΪ1����Fe��O��ԭ����ĿΪ3:4����Fe3O4�ľ�������������������������Χ��6��������Χ�ɵ����������϶���ġ�
(5)�����е��ӿ������ֲ�ͬ��̬�������Ӽ���ٷ���ת�ƣ���Fe3O4�����ܵ��磻��������Ϊa3=��0.42��10-7cm��3��ÿĦ������������Ϊ��56��3+16��4��g=232g����Fe3O4������ܶ�=232g��[NA����0.42��10-7cm��3]=5.2g/cm3��

����Ŀ��������Ԫ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã����û�ѧ����ش��������⣺
���� | ��A | 0 | ||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� |
(1)�ܡ��ݡ�������Ԫ���У����Ӱ뾶��С��˳��Ϊ__>__>__�������ӷ��ţ���
(2)�ڡ��ߡ������ۺ�����������ǿ������˳����(�ѧʽ)___>__>__��
(3)������ݵ�����������ˮ���ﷴӦ�����ӷ���ʽΪ________________��
(4)��˵����ķǽ����ԱȢ�ķǽ�����________���ǿ��������������ʵ�ǣ� _________________�������ӷ���ʽ˵������
(5)�ٺ͢��γɵ�18���ӵĻ��������һ��Ӧ�ù㷺�Ļ���ԭ�ϣ�д���ĵ���ʽ___________��ʵ�����п��ô���������Һ�백��Ӧ�Ʊ��ף���Ӧ�Ļ�ѧ����ʽΪ___________________�������ڴ�����ѹ��¯ˮ�е�������ֹ��¯����ʴ��������1kg�ļɳ�ȥˮ���ܽ��O2________kg��
(6)�����£��ɢ٢����10���ӵĻ������ҿ���Ϊȼ�ϵ�ص�ԭ��֮һ����д�����ڼ��Խ����еĵ缫��Ӧʽ��������____________________������_______________________��

