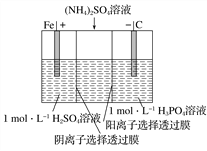
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩœ¬Οφ «‘ΣΥΊ÷ήΤΎ±μΒΡ“Μ≤ΩΖ÷Θ§≤Έ’’‘ΣΥΊΔΌΓΪΔύ‘Ύ±μ÷–ΒΡΈΜ÷ΟΘ§«κ”ΟΜ·―ß”Ο”οΜΊ¥πœ¬Ν–Έ ΧβΘΚ
÷ήΤΎ | ΔώA | 0 | ||||||
1 | ΔΌ | ΔρA | ΔσA | ΔτA | ΔθA | ΔωA | ΔςA | |
2 | ΔΎ | Δέ | Δή | |||||
3 | Δί | Δό | ΔΏ | Δύ | Δα |
(1)ΔήΓΔΔίΓΔΔύ»ΐ÷÷‘ΣΥΊ÷–Θ§άκΉ”ΑκΨΕ¥σ–ΓΒΡΥ≥–ρΈΣ__>__>__Θ®ΧνάκΉ”ΖϊΚ≈Θ©ΓΘ
(2)ΔΎΓΔΔΏΓΔΔύΒΡΉνΗΏΦέΚ§―θΥαΥα–‘”…«ΩΒΫ»θΒΡΥ≥–ρ «(ΧνΜ·―ß Ϋ)___>__>__ΓΘ
(3)ΔόΒΞ÷ ”κΔίΒΡΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ________________ΓΘ
(4)ΡήΥΒΟςΔύΒΡΖ«Ϋπ τ–‘±»ΔαΒΡΖ«Ϋπ τ–‘________Θ®ΧνΓΑ«ΩΓ±ΜρΓΑ»θΓ±Θ©ΒΡ ¬ Β «ΘΚ _________________Θ®”ΟάκΉ”ΖΫ≥Χ ΫΥΒΟςΘ©ΓΘ
(5)ΔΌΚΆΔέ–Έ≥…ΒΡ18ΒγΉ”ΒΡΜ·ΚœΈοΦΉ «“Μ÷÷”Π”ΟΙψΖΚΒΡΜ·ΙΛ‘≠ΝœΘ§–¥≥ωΦΉΒΡΒγΉ” Ϋ___________Θ§ Β―ι “÷–Ω…”Ο¥Έ¬»ΥαΡΤ»ή“Κ”κΑ±Ζ¥”Π÷Τ±ΗΦΉΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___________________Θ§ΦΉΩ…”Ο”Ύ¥ΠάμΗΏ―ΙΙχ¬·Υ°÷–ΒΡ―θΘ§Ζά÷ΙΙχ¬·±ΜΗ· ¥Θ§άμ¬έ…œ1kgΒΡΦΉΩ…≥ΐ»ΞΥ°÷–»ήΫβΒΡO2________kgΓΘ
(6)≥ΘΈ¬œ¬Θ§”…ΔΌΔΎΉι≥…10ΒγΉ”ΒΡΜ·ΚœΈο““Ω…ΉςΈΣ»ΦΝœΒγ≥ΊΒΡ‘≠Νœ÷°“ΜΘ§«κ–¥≥ω““‘ΎΦν–‘Ϋι÷ ÷–ΒΡΒγΦΪΖ¥”Π ΫΘΚ’ΐΦΪΘΚ____________________ΘΜΗΚΦΪ_______________________ΓΘ
ΓΨ¥πΑΗΓΩ S2- O2- Na+ H2SO4 H2CO3 H2SiO3 2Al+2OH-+2H2O=2AlO2-+3H2Γϋ »θ Cl2+S2-=S+2Cl- ![]() 2NH3+NaClO®TN2H4+NaCl+H2O 1 2O2+4H2O +8e-=8OH- CH4+10OH-Θ≠8e-=CO32-+7H2O
2NH3+NaClO®TN2H4+NaCl+H2O 1 2O2+4H2O +8e-=8OH- CH4+10OH-Θ≠8e-=CO32-+7H2O
ΓΨΫβΈωΓΩ≤Έ’’‘ΣΥΊΔΌΓΪΔύ‘Ύ±μ÷–ΒΡΈΜ÷ΟΩ…≈–ΕœΖ÷±π «HΓΔCΓΔNΓΔOΓΔNaΓΔAlΓΔSiΓΔSΓΔClΓΘ‘ρ
(1)ΔήΓΔΔίΓΔΔύ»ΐ÷÷‘ΣΥΊ÷–ΝράκΉ”ΚΥΆβ”–3ΗωΒγΉ”≤ψΘ§άκΉ”ΑκΨΕΉν¥σΓΘ―θ‘ΣΥΊΒΡ‘≠Ή”–ρ ΐ–Γ”ΎΡΤ‘ΣΥΊΘ§‘ρ―θάκΉ”ΑκΨΕ¥σ”ΎΡΤάκΉ”ΑκΨΕΘ§“ρ¥ΥάκΉ”ΑκΨΕ¥σ–ΓΒΡΥ≥–ρΈΣS2-ΘΨO2-ΘΨNa+ΓΘ(2)ΔΎΓΔΔΏΓΔΔύΒΡΖ«Ϋπ τ–‘«Ω»θΥ≥–ρ «SΘΨCΘΨSiΘ§‘ρΉνΗΏΦέΚ§―θΥαΒΡΥα–‘”…«ΩΒΫ»θΒΡΥ≥–ρ «H2SO4ΘΨH2CO3ΘΨH2SiO3ΓΘ(3) ΔόΒΞ÷ ”κΔίΒΡΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·Έο«β―θΜ·ΡΤ»ή“ΚΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ2Al+2OH-+2H2O=2AlO2-+3H2ΓΘ(4)¬»ΤχΡήΑ―ΝράκΉ”÷ΟΜΜ≥ωά¥Θ§ΥΒΟςSΒΡΖ«Ϋπ τ–‘±»ClΒΡΖ«Ϋπ τ–‘»θΘ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣCl2+S2-=S+2Cl-ΓΘ(5)ΔΌΚΆΔέ–Έ≥…ΒΡ18ΒγΉ”ΒΡΜ·ΚœΈοΦΉ «N2H4Θ§ΒγΉ” ΫΈΣ![]() ΘΜ Β―ι “÷–Ω…”Ο¥Έ¬»ΥαΡΤ»ή“Κ”κΑ±Ζ¥”Π÷Τ±ΗΦΉΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ2NH3+NaClOΘΫN2H4+NaCl+H2OΘΜN2H4ΒΡ―θΜ·≤ζΈο «ΒΣΤχΚΆΥ°Θ§Φ¥1molN2H4 ß»Ξ4molΒγΉ”Θ§ΗυΨίΒγΉ”ΒΟ ß ΊΚψΩ…÷ΣœϊΚΡ―θΤχ «1molΓΘ“ρ¥Υ1kgΒΡΦΉΩ…≥ΐ»ΞΥ°÷–»ήΫβΒΡO2
ΘΜ Β―ι “÷–Ω…”Ο¥Έ¬»ΥαΡΤ»ή“Κ”κΑ±Ζ¥”Π÷Τ±ΗΦΉΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ2NH3+NaClOΘΫN2H4+NaCl+H2OΘΜN2H4ΒΡ―θΜ·≤ζΈο «ΒΣΤχΚΆΥ°Θ§Φ¥1molN2H4 ß»Ξ4molΒγΉ”Θ§ΗυΨίΒγΉ”ΒΟ ß ΊΚψΩ…÷ΣœϊΚΡ―θΤχ «1molΓΘ“ρ¥Υ1kgΒΡΦΉΩ…≥ΐ»ΞΥ°÷–»ήΫβΒΡO2![]() ΓΘ(6)≥ΘΈ¬œ¬Θ§”…ΔΌΔΎΉι≥…10ΒγΉ”ΒΡΜ·ΚœΈο““ «ΦΉΆιΘ§ΦΉΆι‘ΎΗΚΦΪΆ®»κΘ§ΖΔ…ζ ß»ΞΒγΉ”ΒΡ―θΜ·Ζ¥”ΠΓΘ‘ΎΦΉΆι‘ΎΦν–‘Ϋι÷ ÷–ΒΡΒγΦΪΖ¥”Π ΫΈΣ’ΐΦΪΘΚ2O2+4H2O+8e-=8OH-ΘΜΗΚΦΪΘΚCH4+10OH-Θ≠8e-=CO32-+7H2OΓΘ
ΓΘ(6)≥ΘΈ¬œ¬Θ§”…ΔΌΔΎΉι≥…10ΒγΉ”ΒΡΜ·ΚœΈο““ «ΦΉΆιΘ§ΦΉΆι‘ΎΗΚΦΪΆ®»κΘ§ΖΔ…ζ ß»ΞΒγΉ”ΒΡ―θΜ·Ζ¥”ΠΓΘ‘ΎΦΉΆι‘ΎΦν–‘Ϋι÷ ÷–ΒΡΒγΦΪΖ¥”Π ΫΈΣ’ΐΦΪΘΚ2O2+4H2O+8e-=8OH-ΘΜΗΚΦΪΘΚCH4+10OH-Θ≠8e-=CO32-+7H2OΓΘ

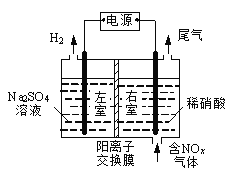
 Κ°ΦΌά÷‘Α±±Ψ©ΫΧ”ΐ≥ωΑφ…γœΒΝ–¥πΑΗ
Κ°ΦΌά÷‘Α±±Ψ©ΫΧ”ΐ≥ωΑφ…γœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ[Μ·―ß-―Γ–ό3ΘΚΈο÷ ΫαΙΙ”κ–‘÷ ]
Χζ―θΧε «“Μ÷÷¥≈–‘≤ΡΝœΘ§ΨΏ”–ΙψΖΚΒΡ”Π”ΟΓΘ -
(1)ΜυΧ§Χζ‘≠Ή”ΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ[Ar]_______ΓΘ
(2)ΙΛ“Β÷Τ±ΗΧζ―θΧε≥Θ Ι”ΟΥ°ΫβΖ®Θ§÷Τ±Η ±≥ΘΦ”»κΡρΥΊ[CO(NH2)2 ]ΓΔ¥ΉΥαΡΤΒ»Φν–‘Έο÷ ΓΘΡρΥΊΖ÷Ή”÷–ΥΡ÷÷≤ΜΆ§‘ΣΥΊΒΡΒγΗΚ–‘”…¥σ÷Ν–ΓΒΡΥ≥–ρ «____________ΘΜ¥ΉΥαΡΤ÷–ΧΦ‘≠Ή”ΒΡ‘”Μ·άύ–Ά «_________ΓΘ
(3)ΙΛ“Β÷Τ±ΗΧζ―θΧε“≤Ω… Ι”Ο≥ΝΒμΖ®Θ§÷Τ±Η ±≥ΘΦ”»κΑ±Θ®NH3)ΓΔΝΣΑ±(N2H4)Β»»θΦνΓΘ±»Ϋœœ¬±μ÷–Α±(NH3)ΓΔΝΣΑ±(N2H4)ΒΡ»έΖ–ΒψΘ§Ϋβ ΆΤδΗΏΒΆΒΡ÷ς“Σ‘≠“ρ________ΓΘ
N2H4 | NH3 | |
»έΒψ/Γφ | 2 | -77.8 |
Ζ–Βψ/Γφ | 113.5 | -33.5 |
(4)œ¬ΆΦ «¥”Χζ―θΧεάκΉ”ΨßΧεFe3O4÷–Θ§»Γ≥ωΒΡΡήΧεœ÷ΤδΨßΧεΫαΙΙΒΡ“ΜΗωΝΔΖΫΧεΘ§‘ρΨßΧε÷–ΒΡ―θάκΉ” «ΖώΙΙ≥…ΝΥΟφ–ΡΝΔΖΫΉνΟήΕ―Μΐ______(ΧνΓΑ «Γ±ΜρΓΑΖώΓ±Θ©Θ§ΗΟΝΔΖΫΧε «≤Μ «Fe3O4ΒΡΨßΑϊ______(ΧνΓΑ «Γ±ΜρΓΑΖώΓ±Θ©Θ§ΝΔΖΫΧε÷–»ΐΦέΧζάκΉ”¥Π”Ύ―θάκΉ”Έß≥…ΒΡ_____Ω’œΕ(ΧνΩ’ΦδΫαΙΙΘ©ΓΘ
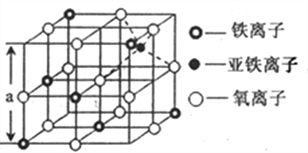
(5)Ϋβ ΆΗΟFe3O4ΨßΧεΡήΒΦΒγΒΡ‘≠“ρ________Θ§ΗυΨί…œΆΦΦΤΥψFe3O4ΨßΧεΒΡΟήΕ»_____gcm-3ΓΘ (ΆΦ÷–a=0.42nmΘ§ΦΤΥψΫαΙϊ±ΘΝτΝΫΈΜ”––ß ΐΉ÷Θ©