��Ŀ����
��8�֣�����ͼ��ʾ����A�г���1mol X��1mol Y����B�г���2mol X��2mol Y����ʼʱA��B�������Ϊa L������ͬ�¶Ⱥ��д��������£��������и��Է�����Ӧ��
X(g)+Y(g) ?2Z(g)+W(g)������ӦΪ���ȷ�Ӧ���ﵽƽ��ʱA���������Ϊ1.2a L��

(1) A��X��ת���ʦ�A=
(2) A��B��X��ת���ʦ�A ��B���� >��< �� = ��
(3) ��K��һ��ʱ���ִﵽƽ��ʱA�����Ϊ L����ͨ��������������Բ��ƣ�
(4) ��(3)�ﵽƽ���ͬʱ�ȷ�����A��B���¶ȣ��ﵽ��ƽ���A����� ������С�䣩
X(g)+Y(g) ?2Z(g)+W(g)������ӦΪ���ȷ�Ӧ���ﵽƽ��ʱA���������Ϊ1.2a L��

(1) A��X��ת���ʦ�A=
(2) A��B��X��ת���ʦ�A ��B���� >��< �� = ��
(3) ��K��һ��ʱ���ִﵽƽ��ʱA�����Ϊ L����ͨ��������������Բ��ƣ�
(4) ��(3)�ﵽƽ���ͬʱ�ȷ�����A��B���¶ȣ��ﵽ��ƽ���A����� ������С�䣩
��1��40%��2��> ��3��2.6a L ��4����С
��1�� X(g)+Y(g) ?2Z(g)+W(g)
��ʼ����mol�� 1 1 0 0
ת������mol�� x x 2x x
ƽ������mol�� 1-x 1-x 2x x
��Ϊ���֮�������ʵ���֮��
����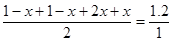
���x��0.4
��ת������40��
��2��A���ֺ�ѹ��B���ֺ��ݡ�����Ӧ���������ķ�Ӧ��ѹǿ��ת���ʵ͡��ڷ�Ӧ������B�е�ѹǿ����A�еġ�����A�е�ת���ʴ���B�е�ת���ʡ�
��3����֮���൱�ڱ��ֺ�ѹ�����ƽ���A�ǵ�Ч�ģ���ת��������ͬ�ġ���ƽ��ʱ���������1.2aL��3��3.6aL������A�����Ϊ3.6aL��aL��2.6aL��
��4����Ӧ�Ƿ��ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�����ƶ��������С��
��ʼ����mol�� 1 1 0 0
ת������mol�� x x 2x x
ƽ������mol�� 1-x 1-x 2x x
��Ϊ���֮�������ʵ���֮��
����
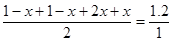
���x��0.4
��ת������40��
��2��A���ֺ�ѹ��B���ֺ��ݡ�����Ӧ���������ķ�Ӧ��ѹǿ��ת���ʵ͡��ڷ�Ӧ������B�е�ѹǿ����A�еġ�����A�е�ת���ʴ���B�е�ת���ʡ�
��3����֮���൱�ڱ��ֺ�ѹ�����ƽ���A�ǵ�Ч�ģ���ת��������ͬ�ġ���ƽ��ʱ���������1.2aL��3��3.6aL������A�����Ϊ3.6aL��aL��2.6aL��
��4����Ӧ�Ƿ��ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�����ƶ��������С��

��ϰ��ϵ�д�
�����Ŀ
 Z(g)����60s�ﵽƽ�⣬����0.3 mol Z��������ȷ����( )
Z(g)����60s�ﵽƽ�⣬����0.3 mol Z��������ȷ����( ) 2NH3(g��;
2NH3(g��; ,10minʱ�����ʵ�Ũ�Ȳ��ٱ仯�����NH3�����ʵ���Ϊ0.4mol��
,10minʱ�����ʵ�Ũ�Ȳ��ٱ仯�����NH3�����ʵ���Ϊ0.4mol��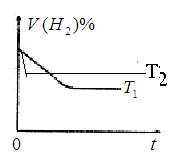

 CO2(g)+H2(g)��CO2��ƽ�����ʵ���Ũ��c(CO2)���¶�T�Ĺ�ϵ��ͼ��ʾ������˵��������ǣ� ��
CO2(g)+H2(g)��CO2��ƽ�����ʵ���Ũ��c(CO2)���¶�T�Ĺ�ϵ��ͼ��ʾ������˵��������ǣ� ��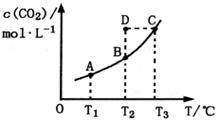
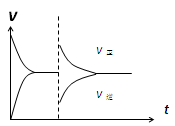
 nC��s��+D��g����ƽ�⡣��ά���¶Ȳ��䣬����ѹǿ����û�������ƽ����Է����������䣬�������ж���ȷ����( )
nC��s��+D��g����ƽ�⡣��ά���¶Ȳ��䣬����ѹǿ����û�������ƽ����Է����������䣬�������ж���ȷ����( )
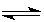 2C(g)��D(g)����һ���¶ȡ�����̶����ܱ������н��У�������������˵��������Ӧ�ﵽƽ��״̬����
2C(g)��D(g)����һ���¶ȡ�����̶����ܱ������н��У�������������˵��������Ӧ�ﵽƽ��״̬����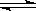 2SO3.5min��ﵽƽ��,�����������SO31.6mol.
2SO3.5min��ﵽƽ��,�����������SO31.6mol.