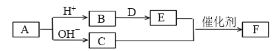
��Ŀ����
����Ŀ��(1)��ϵͳ������������
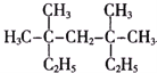 __________________.
__________________.
(2)��Ҫ��д���л���Ľṹ��ʽ��֧��ֻ��һ���һ�����Է���������С������________.
(3)�ǻ��ĵ���ʽΪ________.
(4)![]() ��Br2����1��4�ӳɷ�Ӧ�Ļ�ѧ����ʽΪ________.
��Br2����1��4�ӳɷ�Ӧ�Ļ�ѧ����ʽΪ________.
(5)����˵�������Ϊ________(��ѡ����ĸ)
a.ʵ������ȡ��ϩʱ�����¶ȼƵ�ˮ�������Һ������
b.�����������ڵ���HNO3��AgNO3��Һ�У��ɼ�������������к��е���ԭ��
c.�������NaOH�Ĵ���Һ�������ȣ�������������ֱ��ͨ������KMnO4��Һ�����鷴Ӧ�Ƿ���������ϩ
d.��ͭ˿�Ƴ�����״���ھƾ����ϼ��ȱ�ں���������ʢ����ˮ�Ҵ����Թ��У���ˮ�Ҵ��ɱ�����Ϊ��ȩ
(6)A��B�ķ���ʽ��ΪC2H4Br2��A�ĺ˴Ź�������ֻ��һ�����շ壬��A�Ľṹ��ʽΪ___����Ԥ��B�ĺ˴Ź���������___�����շ塣
���𰸡�3��3��5��5���ļ����� 

![]() +Br2��
+Br2�� bc BrCH2CH2Br 2
bc BrCH2CH2Br 2
��������
��1��������������ԭ���л���Ϊ3��3��5��5���ļ����飻
��2��֧��ֻ��һ���һ�������Է���������С������Ϊ3���һ����飬���ṹ��ʽΪ ��
��
��3���ǻ��ĵ���ʽΪ ��
��
��4��![]() ����1��4�ӳɷ�Ӧ�ķ���ʽΪ
����1��4�ӳɷ�Ӧ�ķ���ʽΪ![]() +Br2��
+Br2�� ��
��
��5��a��ʵ�����Ʊ���ϩ���䷴Ӧ����ʽΪCH3CH2OH![]() CH2=CH2����H2O���¶ȼƵ�ˮ�������Һ�����£���a˵����ȷ��
CH2=CH2����H2O���¶ȼƵ�ˮ�������Һ�����£���a˵����ȷ��
b����������ClΪԭ�ӣ�������������Ҫ��Clԭ��ת����Cl�����������鷢��ˮ�ⷴӦ����ȥ��Ӧ����Ҫ��NaOHˮ��Һ��NaOH����Һ�н��У�Ȼ����������ữ��Һ�����μ�AgNO3��Һ���۲������ɫ����b����
c�������������п��ܺ��д�����Ҳ��ʹ���Ը��������Һ��ɫ����ʵ��������ţ���c˵������
d���ھƾ��������գ�����2Cu��O2 ![]() 2CuO��Ȼ��CuO��CH3CH2OH
2CuO��Ȼ��CuO��CH3CH2OH ![]() CH3CHO��Cu��H2O����d˵����ȷ��
CH3CHO��Cu��H2O����d˵����ȷ��
��6��A�ĺ˴Ź�������ֻ��һ�����շ壬˵��ֻ��һ���⣬��A�Ľṹ��ʽΪBrCH2CH2Br����B�Ľṹ��ʽΪCH3CHBr2����2�ֲ�ͬ����ԭ�ӣ����˴Ź���������2�ַ塣

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д�����Ŀ��������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ����������ͼAװ���Ʊ�����������

(1)��ʵ����������ͺ�18O���Ҵ����ã��÷�Ӧ�Ļ�ѧ����ʽ�ǣ�______����̲IJ��õ�ʵ��װ�ò�ͬ����װ���в��������θ���ܣ���������______��
(2)Ϊ��֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý���������4��ʵ�飮ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թ����ٲ��л���ĺ�ȣ�ʵ���¼���£�
ʵ���� | �Թ�I�е��Լ� | �Թ�II�е��Լ� | �л���ĺ��/cm |
A | 2mL�Ҵ���1mL���� 1mL18molL-1Ũ���� | ����Na2CO3 | 3.0 |
B | 2mL�Ҵ���1mL���� | 0.1 | |
C | 2mL�Ҵ���1mL���� 3mL2molL-1H2SO4 | 0.6 | |
D | 2mL�Ҵ���1mL���ᡢ���� | 0.6 |
��ʵ��D��Ŀ������ʵ��C����գ�֤��H+��������Ӧ���д����ã�ʵ��D��Ӧ��������������Ũ�ȷֱ���______mL��______molL-1��
������ʵ��______(��ʵ����)�����ݣ������Ʋ��ŨH2SO4����ˮ����������������IJ��ʡ�
(3)����������90g���Ҵ�138g����������Ӧ�õ�88g�����������Լ���÷�Ӧ�IJ���Ϊ______��
(4)Ϊ������÷�Ӧ��ס�����λͬѧ�ֱ��������ͼ�ס�������װ��(��ͬѧ����Ӧ�����ȴ�����ñ���̼������Һ��ȡ��ƿ�еIJ���)������Ϊ���������______��