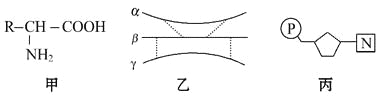
��Ŀ����
����Ŀ����1��3.6 g H2O�����ʵ�����____������ˮ���ӵ���Ŀ��_____��������ԭ�ӵ����ʵ�����_____��
��2��1.5 mol CO2��______g CH4�ڱ�״����ռ����ͬ������������Ϊ________��
��3��100 mL����A2��50 mL����B2ǡ����ȫ��Ӧ����100 mL����C�����������ͬ�����²ⶨ����C�Ļ�ѧʽΪ________��
��4������mgijX2���壬����Ħ������ΪM g/mol�������ӵ�������NA��ʾ������������ʵ���Ϊ______mol��һ��Xԭ�ӵ�����Ϊ_______g���������ڱ�״���µ����Ϊ______L��
���𰸡�0.2 mol0.2��6.02��10230.4mol2433.6 LA2Bm/M![]() 22.4m/M
22.4m/M
��������
��1��3.6 g H2O�����ʵ�����3.6g��18g/mol��0.2mol������ˮ���ӵ���Ŀ��0.2��6.02��1023��������ԭ�ӵ����ʵ�����0.2mol��2��0.4mol��
��2�����ݰ����ӵ����ɿ�֪��ͬ���������֮�������ʵ���֮�ȣ�1.5 mol CO2��CH4�ڱ�״����ռ����ͬ�������˵����������ʵ���Ҳ��1.5mol��������1.5mol��16g/mol��24g�������Ϊ1.5mol��22.4L/mol��33.6 L��
��3��100 mL����A2��50 mL����B2ǡ����ȫ��Ӧ����100 mL����C�����������ͬ�����²ⶨ��������ݰ����ӵ����ɿ�֪������������ʵ���֮����2��1��2����˷�Ӧ�ķ���ʽΪ2A2+B2��2C�������ԭ���غ��֪C�Ļ�ѧʽΪA2B��
��4��mgijX2���壬����Ħ������ΪM g/mol�������������ʵ���Ϊm/M mol���������ڱ�״���µ����Ϊm/M mol��22.4L/mol��22.4m/M L���������к���Xԭ�ӵĸ�����![]() ������1��Xԭ�ӵ�����Ϊ
������1��Xԭ�ӵ�����Ϊ![]() ��
��

 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�