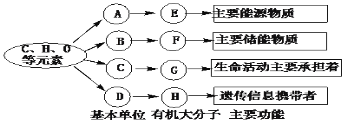
��Ŀ����
����Ŀ����ͼ�ס��ҡ���Ϊ������������ػ������Ϊһ�������������������������γɵĵ����ʷ��ӣ�����271�������ᣬͼ��ÿ�����߱�ʾ���������(-SH)�����γ�һ�������(-S-S-)�����������������ȷ����
A. ��Ϊ����ҵĻ�����λ����������ຬ��20�ּ�
B. �ɲ�ͬ�ļ��γ��Һ���Է���������ԭ������4 832
C. ����Ҫ������ϸ�����У������ҵ�����ϳ��о�����Ҫ����
D. ������е�RΪC3H5O2�����������Ӽ��γɵĻ������к���16��H
���𰸡�
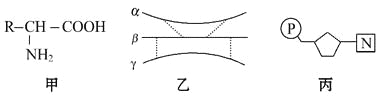
�����������ǰ����ᣬ���ǵ����ʡ���Ϊ����ҵĻ�����λ�����ɵ����ʵİ�����Լ20�֣�A����ȷ�������ᾭ����ˮ�����γɵ����ʣ������ϻ���-SH�������γ�һ����������ɲ�ͬ�ļ��γ��Һ���Է�������ԭ�������ˣ�271��3����18��4��2��4832��B����ȷ������ʾ�����ᣬ�������Ǻ���������������ᣬ�����ֺ���������������ϸ�����У��ٲ��ִ����������塢Ҷ�����У�Ҳ���ٲ��ִ�����ϸ�����У����վ����˿���ϸ���ˡ�������Ƶ����ʵĺϳɣ��������û�й�ϵ��������ֻ�Ǻϳ�DNA��RNA��ԭ�ϣ�C�����������е�RΪC3H5O2����ķ���ʽΪC5H9O4N�������Ӽ���һ��ˮ�γɵĻ��������ʽΪC10H16O7N�������������Ӽ��γɵĻ������к���16��H��D����ȷ��

����Ŀ��2013��9�£��й��������е������������ص������������������ӱ������ϵȵصĿ�����Ⱦ��Ϊ6��������Ⱦ�������ض���Ⱦ������β����ȼú����������ȡů�ŷŵ�CO2�ȶ��������γɵ�ԭ��
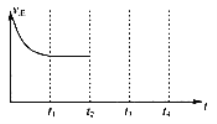
��1������β����������Ҫԭ��Ϊ��2NO(g)��2CO(g) ![]() N2(g)��2CO2(g) ��H<0����һ���¶��£���һ����̶����ܱ������г���һ������NO��CO��t1 ʱ�̴ﵽƽ��״̬��
N2(g)��2CO2(g) ��H<0����һ���¶��£���һ����̶����ܱ������г���һ������NO��CO��t1 ʱ�̴ﵽƽ��״̬��
�����жϸ÷�Ӧ�ﵽƽ��״̬�ı�־��____________________��
A���ڵ�λʱ��������1mol CO2��ͬʱ������1mol CO B�����������ܶȲ��ٸı�
C����������ƽ����Է����������ٸı� D����������ѹǿ���ٱ仯
����t2ʱ�̣����������ݻ�Ѹ������ԭ����2�����������������������£�t3ʱ�̴ﵽ�µ�ƽ��״̬��֮���ٸı�������������ͼ�в��仭����t2 ��t4 ʱ������Ӧ������ʱ��ı仯���ߣ�_____________
��2���ı�ú�����÷�ʽ�ɼ��ٻ�����Ⱦ��ͨ���ɽ�ˮ����ͨ�����ȵ�̼�õ�ˮú����1.2g ̼��ȫ��Ӧ����������13.13kJ.
�ٸ÷�Ӧ���Ȼ�ѧ����ʽΪ______________________________________________
��ú���������в������к�����H2S����������Na2C03��Һ���գ��÷�Ӧ�����ӷ���ʽΪ___________________________________________________������֪��H2S�� ![]() ��
�� ![]() ��H2CO3��
��H2CO3�� ![]() ��
�� ![]() ��
��
��3����֪��Ӧ��CO(g)��H2O(g) ![]() CO2(g)��H2(g)���ֽ���ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������н��з�Ӧ���õ������������ݣ�
CO2(g)��H2(g)���ֽ���ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������н��з�Ӧ���õ������������ݣ�
ʵ�� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | ��ƽ������ʱ��/min | ||
CO | H2O | H2 | CO | 0 | ||
1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
3 | 900 | a | b | c | d | t |
��ʵ��1������ƽ�ⳣ��K=______________������С������λ����
��ʵ��3�У���ƽ��ʱ��CO��ת���ʴ���ˮ��������a��b��������Ĺ�ϵ��__________��
�۸÷�Ӧ�ġ�H ______0(�����������������900��ʱ������һ��ʵ�飬�ڴ������м���l0mol CO��5mo1 H2O��2mo1 CO2��5mol H2�����ʱv��___________v�棨�������������������������
����Ŀ��ͼ1��ʾ�ĵ�ѭ������̬ϵͳ����ѭ������Ҫ��ɲ��֣������Ӿ��˵�ѭ���е�����ת����
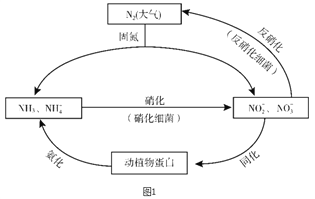
��1�������ͼ�ж�����˵����ȷ����________������ĸ��ţ���
A. �̵������У�N2ֻ��������
B. ������ϸ�������·���������������Ҫ������������
C. �����������������ֲ��˹��̵��Ե�ѭ����ɵ�Ӱ��
D. ͬ�������������У���Ԫ�ؾ�������ת�����л���
��2�����������У�NH3ת����HNO2�ķ�Ӧ�Ļ�ѧ����ʽΪ_______��
��3�������������У�CH3OH����Ϊ��Ӧ�Ļ�ԭ�����뽫�÷�Ӧ�����ӷ���ʽ����������5CH3OH + 6NO3- ![]() N2�� + 4HCO3- +��______+��
N2�� + 4HCO3- +��______+��
��4�������±����ݽ��й��㣬д����ҵ�ϳɰ���Ӧ���Ȼ�ѧ����ʽ��_______��
���ۼ� | N��N | H��H | N��H |
�Ͽ�1mol���ۼ�����������kJ�� | 946 | 436 | 391 |
��5����ⷨ�ϳɰ�����ԭ��ת���ʴ������ߣ��������洫ͳ�Ĺ�ҵ�ϳɰ����ա���ⷨ�ϳɰ�������ԭ����װ����ͼ2��ͼ3��ʾ��
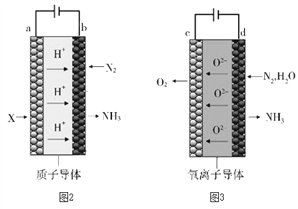
��ͼ2�У�a�缫��ͨ���XΪ_______��
��ͼ3�У�d�缫�ϵĵ缫��ӦʽΪ_______��
����ͼ2��ͼ3װ�õ�ͨ��ʱ����ͬ������ǿ����ȣ����Ч�ʷֱ�Ϊ80%��60%��������װ���в������������ʵ���֮��Ϊ_______��