��Ŀ����
��13�֣�
��ˮ�Ǿ�Ļ�ѧ��Դ���⡣
�Ӻ�ˮ�п�����ȡ�ȡ��塢���±��Ԫ�ء�
��1��Cl2�ĵ���ʽ�� ��
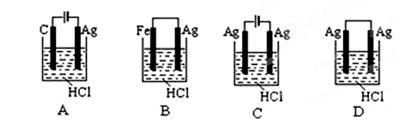
��2����֪��X2 (g)+H2(g) 2HX(g) (X2��ʾCl2��Br2��I2)��
2HX(g) (X2��ʾCl2��Br2��I2)��
��ͼ��ʾƽ�ⳣ��K���¶�t�Ĺ�ϵ��
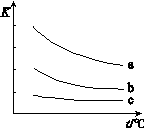
�٦�H ��ʾX2��H2��Ӧ���ʱ䣬��H 0�����������������������
�� ����a��ʾ���� ���Cl2������Br2����I2������H2��ӦʱK��t�Ĺ�ϵ��
��ˮ�������й㷺��Ӧ��ǰ��������ǰ��Ժ�ˮ����Ԥ������
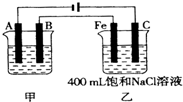
��1��ͨ����������K2SO4��Al2(SO4)3��24H2O�����������������Ƕȡ�����ˮ������ӷ���ʽ�� ��
��2������ͼ��ʾNaClO�ķ���װ�öԺ�ˮ�������������崦����
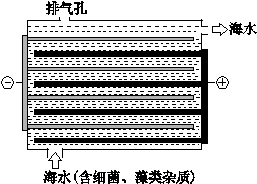
�� װ������NaClת��ΪNaClO�Ļ�ѧ����ʽ�� ��
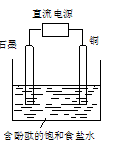
�� ��ˮ�к���Ca2+��Mg2+��HCO3�����������ӣ�����������װ�õ������ײ���ˮ��������Ҫ�ɷ���Mg(OH)2��CaCO3������CaCO3�����ӷ���ʽ�� ��
�� ��ÿ��5-10 min����һ�ε缫���ԣ�����Ч�ؽ�������ĽṸ���⡣
���õ缫��Ӧʽ����ϱ�Ҫ�����ֽ��н��� ��
��ˮ�Ǿ�Ļ�ѧ��Դ���⡣
�Ӻ�ˮ�п�����ȡ�ȡ��塢���±��Ԫ�ء�
��1��Cl2�ĵ���ʽ�� ��
��2����֪��X2 (g)+H2(g)
 2HX(g) (X2��ʾCl2��Br2��I2)��
2HX(g) (X2��ʾCl2��Br2��I2)�� ��ͼ��ʾƽ�ⳣ��K���¶�t�Ĺ�ϵ��

�٦�H ��ʾX2��H2��Ӧ���ʱ䣬��H 0�����������������������
�� ����a��ʾ���� ���Cl2������Br2����I2������H2��ӦʱK��t�Ĺ�ϵ��
��ˮ�������й㷺��Ӧ��ǰ��������ǰ��Ժ�ˮ����Ԥ������
��1��ͨ����������K2SO4��Al2(SO4)3��24H2O�����������������Ƕȡ�����ˮ������ӷ���ʽ�� ��
��2������ͼ��ʾNaClO�ķ���װ�öԺ�ˮ�������������崦����

�� װ������NaClת��ΪNaClO�Ļ�ѧ����ʽ�� ��
�� ��ˮ�к���Ca2+��Mg2+��HCO3�����������ӣ�����������װ�õ������ײ���ˮ��������Ҫ�ɷ���Mg(OH)2��CaCO3������CaCO3�����ӷ���ʽ�� ��
�� ��ÿ��5-10 min����һ�ε缫���ԣ�����Ч�ؽ�������ĽṸ���⡣
���õ缫��Ӧʽ����ϱ�Ҫ�����ֽ��н��� ��
��13�֣�
��1��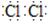 ��1�֣���
��1�֣���
��2���� ����2�֣��� �� Cl2��2�֣���
��1��Al3++3H2O Al(OH)3+3H+ ��2�֣���
Al(OH)3+3H+ ��2�֣���
��2���� 2NaCl+2H2O 2NaOH+Cl2+H2�� ��1�֣���
2NaOH+Cl2+H2�� ��1�֣���
2NaOH+Cl2=NaClO+NaCl+H2O ��1�֣���
�� Ca2+��HCO3-��OH-��CaCO3����H2O ��2�֣���
�������Ṹ���缫���ԣ�������Ϊ��������缫��ӦΪ��2Cl--2e-=Cl2����������������ˮ������Ӧ��Cl2+H2O=HCl+HClO��ʹ�õ缫������Һ�����ԣ��Ӷ���Mg(OH)2��CaCO3�ܽ���ﵽ������Ŀ�ġ� ��2�֣�
��1��
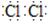 ��1�֣���
��1�֣���
|
��1��Al3++3H2O
 Al(OH)3+3H+ ��2�֣���
Al(OH)3+3H+ ��2�֣�����2���� 2NaCl+2H2O
 2NaOH+Cl2+H2�� ��1�֣���
2NaOH+Cl2+H2�� ��1�֣���2NaOH+Cl2=NaClO+NaCl+H2O ��1�֣���
�� Ca2+��HCO3-��OH-��CaCO3����H2O ��2�֣���
�������Ṹ���缫���ԣ�������Ϊ��������缫��ӦΪ��2Cl--2e-=Cl2����������������ˮ������Ӧ��Cl2+H2O=HCl+HClO��ʹ�õ缫������Һ�����ԣ��Ӷ���Mg(OH)2��CaCO3�ܽ���ﵽ������Ŀ�ġ� ��2�֣�
�����������1�����������е�Clԭ��֮���γ�1�Թ��õ��Ӷԣ����Ե���ʽΪ
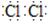
��2������ͼ��֪��ÿ�����߶������¶����ߣ�Kֵ��С�����������¶ȣ�ƽ�������ƶ�������Ӧ�Ƿ��ȷ�Ӧ����H<0��
��ͬһ�¶�ʱ��a���ߵ�Kֵ���˵��±�ص������������ϵ�������ǿ��Cl2��Br2��I2��Cl2����������ǿ�������������������ϵ�������������a���߱�ʾCl2��H2��ӦʱK��t�Ĺ�ϵ��
��1�������е�������ˮ�����������������壬���нϴ����������������Ƕȡ�ˮ������ӷ���ʽΪAl3++3H2O
 Al(OH)3+3H+ ��
Al(OH)3+3H+ ����2���� ��װ���ǵ��װ�ã���ˮ�е��Ȼ��Ƶ�������������������������ƣ�װ���в����ڸ���װ�ã�ʹ���ɵ��������������Ʒ�Ӧ�����˴������ƣ���ѧ����ʽΪ2NaCl+2H2O
 2NaOH+Cl2+H2����2NaOH+Cl2=NaClO+NaCl+H2O��
2NaOH+Cl2+H2����2NaOH+Cl2=NaClO+NaCl+H2O����װ�õ������������ӷŵ磬����������OH-����Ũ�������뺣ˮ�е�HCO3-��Ӧ����CO32-������Ca2+�������̼��Ƴ�������ѧ����ʽΪ��Ca2+��HCO3-��OH-��CaCO3����H2O��
��ÿ��5-10 min����һ�ε缫���ԣ��������������������������ӷŵ�����������2Cl--2e-=Cl2������������ˮ��������ʹ����ᣬCl2+H2O=HCl+HClO��ʹ�õ缫������Һ�����ԣ��Ӷ���Mg(OH)2��CaCO3�ܽ���ﵽ������Ŀ�ġ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ