��Ŀ����
1���ں����ܱ������г�������ʵ����ĵ���������������һ�������·�����Ӧ��H2��g��+I2?2HI��g����H�T-14.9kJ•mol-1���й�������ͼ1��ʾ��ش��������⣺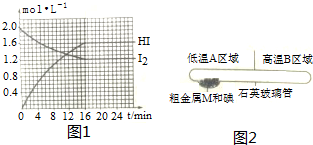
��1�����ڸ������г���1mol I2��g����1 mol H2��g�����ﵽƽ��ʱ�ų���������14.9kJ������ڡ�����С�ڡ����ڡ�����
��2��16minʱ��Ӧ�ﵽƽ�⣬��Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v��H2��Ϊ0.05mol/��L��min����
��3��������Ӧ�ﵽƽ��ʱ��I2��ת����Ϊ40%��
��4������ƽ�ⳣ��K=1.78������ı�ij����ʹƽ�ⳣ��������ı�������ǽ��£�
��5��������ƽ����ϵ�У��ٳ�������ʵ�����H2��g����I2��g����������������ʱ�ٴδﵽƽ�⣬��������ת���ʽ����䣨���������С�����䡱����
��6���ⵥ�ʿ��������ᴿijЩ���۽�����������M��ʾ���йط�Ӧ���£�M��s��+I2��g��?MI2��g����H��0���ֽ���M�к��в����뷴Ӧ���ѻӷ������ʣ���ȡ��ͼ2װ���ᴿ��
�÷�Ӧ��ƽ�ⳣ������ʽΪ$\frac{c��M{I}_{2}��}{c��{I}_{2}��}$����װ�������ᴿ����M��ԭ����M��ⵥ���ڵ�������Ӧ���ڸ�����ƽ�������ַֽ�����M��
���� ��1����Ӧ�ǿ��淴Ӧ���ܽ��г�������
��2��16minʱ��Ӧ�ﵽƽ�⣬ͼ���������HI��ʾ�ķ�Ӧ����=$\frac{��c}{��t}$����Ӧ����֮�ȵ��ڻ�ѧ����ʽ������֮�ȼ��������ķ�Ӧ���ʣ�
��3������ͼ���ȡ�ⵥ�ʵ���ʼŨ�Ⱥ�����Ũ�ȣ����ת����=$\frac{������}{��ʼ��}$��100%���㣻
��4����ͼ���ȡ��ʼ����ƽ��������ϻ�ѧƽ������ʽ��ʽ����ƽ��Ũ�ȣ�ƽ�ⳣ��K=$\frac{������ƽ��Ũ���ݴη��˻�}{��Ӧ��ƽ��Ũ���ݴη��˻�}$��ƽ�ⳣ�����¶ȱ仯���ı�ij����ʹƽ�ⳣ������ֻ�ܸı䷴Ӧ���¶ȣ�
��5����Ӧǰ������������䣬�ٳ�������ʵ�����H2��g����I2��g����ƽ�ⲻ�䣬ת���ʲ��䣻
��6��Ӧ�������Ϣ�еĻ�ѧƽ�⣬���ƽ������ۺ�֪ʶ���з����жϣ�M��s��+I2��g��?MI2��g���ǻ�ѧƽ�⣬M���ѻӷ���������ʣ����ͼʾ��M��λ�ÿ�֪���ᴿ�ǰ�M�͵ⵥ���ڵ����·�Ӧ��������MI2��g�������Ը÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ����������ɢ����������ƽ����������M��I2��g����ʹM�õ��ᴿ��
��� �⣺��1����һ�������·�����Ӧ��H2��g��+I2?2HI��g����H�T-14.9kJ•mol-1����Ӧ�ǿ��淴Ӧ���ܽ��г��ף����ڸ������г���1mol I2��g����1 mol H2��g�����ﵽƽ��ʱ�ų�������С��14.9kJ��
�ʴ�Ϊ��С�ڣ�
��2��16minʱ��Ӧ�ﵽƽ�⣬ͼ���������HI��ʾ�ķ�Ӧ����V��HI��=$\frac{��c}{��t}$=$\frac{1.6mol/L}{16min}$=0.1mol/L•min����Ӧ����֮�ȵ��ڻ�ѧ����ʽ������֮�ȼ��������ķ�Ӧ���ʣ�V��H2��=$\frac{1}{2}$V��HI��=$\frac{1}{2}$��0.1mol/L•min=0.05mol/��L��min����
�ʴ�Ϊ��0.05mol/��L��min����
��3��ͼ�������֪���ⵥ����ʼŨ��c��I2��=2.0mol/L��ƽ��Ũ��c��I2��=1.2mol/L������Ũ��=2.0mol/L-1.2mol/L=0.8mol/L����ⵥ�ʵ�ת����=$\frac{0.8mol/L}{2mol/L}$��100%=40%��
�ʴ�Ϊ��40%��
��4����������ʵ����ĵ�������������ͼ�������֪���ⵥ����ʼŨ��c��I2��=2.0mol/L��ƽ��Ũ��c��I2��=1.2mol/L��c��HI��=1.6mol/L
H2��g��+I2?2HI��g��
��ʼ����mol/L�� 2 2 0
�仯����mol/L�� 0.8 0.8 1.6
ƽ������mol/L�� 1.2 1.2 1.6
ƽ�ⳣ��K=$\frac{1��{6}^{2}}{1.2��1.2}$=1.78
H2��g��+I2?2HI��g����H�T-14.9kJ•mol-1����Ӧ�Ƿ��ȷ�Ӧ��ƽ�ⳣ�����¶ȱ仯�������¶�ƽ��������У�ƽ�ⳣ������
�ʴ�Ϊ��1.78�����£�
��5��������ƽ����ϵ�У��ٳ�������ʵ�����H2��g����I2��g������Ӧ�������������ķ�Ӧ��������������ʱ�ٴδﵽƽ�⣬��ԭ����ƽ��״̬��ͬ�����Ե�������ת���ʲ��䣻
�ʴ�Ϊ�����䣻
��6��M��s��+I2��g��?MI2��g������Ӧ��ƽ�ⳣ��K=$\frac{c��M{I}_{2}��}{c��{I}_{2}��}$����װ�������ᴿ����M��ԭ����M��ⵥ���ڵ�������Ӧ���ڸ�����ƽ�������ַֽ�����M��
�ʴ�Ϊ��$\frac{c��M{I}_{2}��}{c��{I}_{2}��}$��M��ⵥ���ڵ�������Ӧ���ڸ�����ƽ�������ַֽ�����M��
���� ���⿼���˻�ѧƽ���Ӱ�����ط���Ӧ�ã���Ӧ���ʡ�ƽ�ⳣ���ļ��㣬ͼ�������ƽ���ƶ�ԭ����Ӧ���ǹؼ�����Ŀ�Ѷ��еȣ�

| A�� | ÿ����1mol������ת�Ƶ�������Ϊ2NA | |
| B�� | �����¶ȣ���������������ʼӿ죬˵�������¶ȿɼӿ컯ѧ��Ӧ���� | |
| C�� | ����ѹǿ�Ը÷�Ӧ�����ʼ���û��Ӱ�� | |
| D�� | ���ڷ�Ӧ����SO2���壬���Կ���ͨ���۲��������ݵĿ����Ƚ�ϡ��Ũ������Na2S2O3��Ӧ�����ʴ�С |
| A�� | OH-��H2O��F- | B�� | H3O+��NH4+��Cl- | C�� | HF��Ne��H2O | D�� | NH3��NH4+��F- |
| ��� | c��HCl����mol•L-1�� | �¶ȣ��棩 | ״̬ |
| 1 | 2.0 | 25 | ��״ |
| 2 | 2.5 | 30 | ��״ |
| 3 | 2.5 | 50 | ��״ |
| 4 | 2.5 | 50 | ��ĩ״ |
| A�� | 1-2-4-3 | B�� | 1-2-3-4 | C�� | 3-4-2-1 | D�� | 4-3-2-1 |
| A�� | ���������У�̼ԭ�Ӷ���̼̼������ϣ�����ļۼ�������ԭ�ӽ�ϣ���һϵ�л�����ķ���ͨʽΪC2nH2n+2 | |
| B�� | ����������һ�������ɸ�CH2ԭ���ŵ����ʣ�����Ϊͬϵ�� | |
| C�� | ���������У����ڵ�����̼ԭ���п�����ͬһ��ֱ���� | |
| D�� | 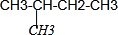 CH4��CH3CH2CH3��Ϊͬϵ�� CH4��CH3CH2CH3��Ϊͬϵ�� |
| A�� | ��ϵͳ�������� ������Ϊ2��7��7-����-3-�һ����� ������Ϊ2��7��7-����-3-�һ����� | |
| B�� | ʵ��֤ʵ ��ʹBr2/CCl4��Һ��ɫ��˵���÷����д��ڶ�����̼̼������̼̼˫�� ��ʹBr2/CCl4��Һ��ɫ��˵���÷����д��ڶ�����̼̼������̼̼˫�� | |
| C�� | DDT�Ľṹ��ʽΪ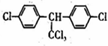 �������������14��̼ԭ�ӹ�ƽ�� �������������14��̼ԭ�ӹ�ƽ�� | |
| D�� |  �ĵ�����CH3-C��C-CH3��CH2=CH-CN �ĵ�����CH3-C��C-CH3��CH2=CH-CN |
 ��
��
