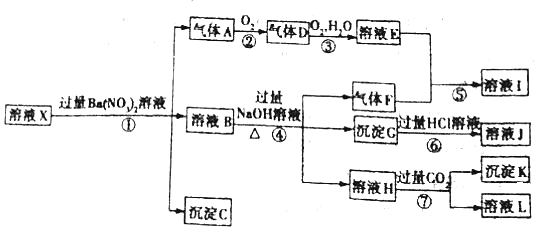
��Ŀ����
����Ŀ��ʵ�����Ʊ���ˮ����ͭ[Cu(HCOO)24H2O]����ʵ�鲽�����¡�
(1)��ʽ̼��ͭ���Ʊ���
a.����i�ǽ�һ����������̼�����ƹ���һ��ŵ��в�����ĥ����Ŀ����______��
b.����ii���ڽ����½���������ֶ�λ���������ˮ�У���Ӧ�¶ȿ�����70�桪80�棬�������_____(дʵ������)��˵���¶ȹ��ߡ�
c.��صĻ�ѧ����ʽ______��
(2)��ˮ����ͭ������Ʊ�������ʽ̼��ͭ��������ձ��У�����һ�����ȵ�����ˮ������μ����������ʽ̼��ͭǡ��ȫ���ܽ⣬���ȹ��˳�ȥ�������������ʣ�Ȼ��������ȴ���ˣ�����������ˮ�Ҵ�ϴ�Ӿ���2��3�����ɣ��õ���Ʒ��
a.��صĻ�ѧ����ʽ______��
b.���ȹ����У�������ȵ�ԭ����______��
c.���Ҵ�ϴ�Ӿ����Ŀ��______��
���𰸡���ϸ����Ͼ��� �к�ɫ�������� ![]()
![]() ��ֹ����ͭ�������� ϴȥ��������ˮ���������ʣ����ټ���ͭ����ʧ
��ֹ����ͭ�������� ϴȥ��������ˮ���������ʣ����ټ���ͭ����ʧ
��������
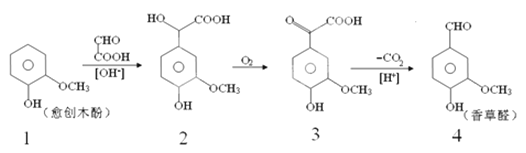
�ŵ�����̼���������ֹ�������ҪҪ��ֽӴ����ܳ�ַ�Ӧ����ʽ̼��ͭ�����ֽ⣬���ɺ�ɫ������ͭ������ͭ��̼�����Ʒ�Ӧ���ɼ�ʽ̼��ͭ������Ԫ���غ���д��ѧ����ʽ��
�Ƽ�ʽ̼��ͭ����ᷴӦ���ɼ���ͭ������Ԫ���غ���д��ѧ����ʽ������ͭ���ܽ�����¶ȵ����߶��������ȹ��ˣ���������������ԭ������֪����ͭ������ˮ���������л��ܼ���
��a.������̼���������ֹ�������ҪҪ��ֽӴ����ܳ�ַ�Ӧ��������ĥ�������ǰ�ҩƷ��ϸ����Ͼ��ȣ��ʴ�Ϊ����ϸ����Ͼ��ȡ�
b.�¶ȹ��ߣ�Cu(OH)2CuCO3��ֽ����ɺ�ɫ������ͭ���ʴ�Ϊ���к�ɫ�������ɡ�
c.����ͭ��̼�����Ʒ�Ӧ���ɼ�ʽ̼��ͭ������Ԫ���ؿ�֪��ѧ����ʽΪ2CuSO4 + 4NaHCO3 = Cu(OH)2CuCO3�� + 3CO2��+ 2Na2SO4 + H2O���ʴ�Ϊ��2CuSO4 + 4NaHCO3 = Cu(OH)2CuCO3�� + 3CO2��+ 2Na2SO4 + H2O��
��a.��ʽ̼��ͭ����ᷴӦ�Ƶ���ˮ����ͭ[Cu(HCOO)24H2O]���壬����Ԫ���ؿ�֪��ѧ����ʽΪCu(OH)2CuCO3 +4HCOOH + 5H2O =2 Cu(HCOO)24H2O + CO2�����ʴ�Ϊ��Cu(OH)2CuCO3 +4HCOOH + 5H2O =2 Cu(HCOO)24H2O + CO2����
b.����ͭ���ܽ�����¶ȵ����߶���������ȴ�����о������������Ͳ��ʣ��������ȹ��ˣ��ʴ�Ϊ����ֹ����ͭ����������
c.����ͭ������ˮ�������ھƾ�������������ˮϴ�ӣ��������Ҵ�����ϴ�ӣ�ϴȥ��������Һ�����ʣ��ʴ�Ϊ��ϴȥ��������ˮ���������ʣ����ټ���ͭ����ʧ��

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�����Ŀ����ѧ�ϳ���ȼ�շ��ⶨ�л���ķ���ʽ�����ַ������ڵ�¯����ʱ�ô���������������Ʒ�����ݲ��������ȷ���л������ɡ���ͼ��ʾ������ȼ�շ�ȷ���л������ʽ�ij���װ�á�
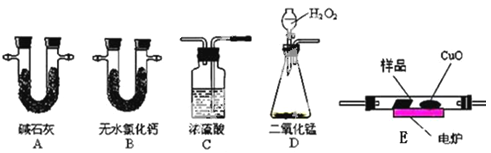
ʵ��̽��С���ȡһ����������Ʒ��ֻ��C��H��O����Ԫ���е����ֻ����֣�������ʵ��,ͨ���ⶨ������CO2��ˮ������ȷ���л������ɣ���ش��������⣺
��1��C��Ũ����������dz�ȥ�����е�ˮ������ʵ��װ�õ�����˳��Ӧ�ǣ�___________��ÿ��װ��ֻ��һ�Σ���
��2��ʵ�����ݼ�¼�ʹ���
������ʵ����� | ȼ���л�������� | �� | �� | ||
ʵ��ǰ���� | ʵ������� | ʵ��ǰ���� | ʵ������� | ||
1 | m1 | m2 | m3 | m4 | m5 |
�ϱ��Т١��ڷֱ�ָ�ĸ�װ�ã�____________ �� _____________��
��3����ʵ��ȷ��ȡ4.4 g��Ʒ����ȼ�պ��ò���CO28.8 g��ˮ����3.6g��Ҫȷ�����л���ķ���ʽ��������֪����������________��
��4����ͬ�����£������л�����������������Է�������Ϊ22�������ĺ˴Ź����������������壬��ǿ�ȱ�Ϊ3:1����ͨ������ȷ�����л���Ľṹ��ʽ___________������л�����Է���������ͬ������һ�ȴ�����_____�֡�
����Ŀ������ʵ���У�������ͷ���������У��ҷ��������������
ʵ��Ŀ�� | ������ | ������ | |
A | ��ȥ���������е��������� | �ӱ��� | ���� |
B | ����̼���ƺ�̼��������Һ | �ֱ�μӳ���ʯ��ˮ | �ֱ��������Һ |
C | �������������Ƿ����� | ���� | ���������ữ�� |
D | �Ƚ���Ԫ�ء���Ԫ�صķǽ�����ǿ�� | �ֱ�����Ȼ��⡢�⻯�⣬�Ƚ����ȶ��� | �ڵ��۵⻯����ֽ�ϵμ���ˮ |
A.AB.BC.CD.D