��Ŀ����
����Ŀ����.NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ��NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺
(1)��ͬ�����£�0.1 mol��L��1NH4Al(SO4)2��c(NH4+)________(������������������������С����)0.1 mol��L��1NH4HSO4��c(NH4+)��
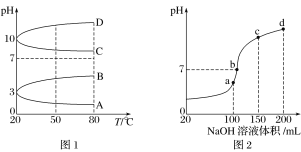
(2)��ͼ1��0.1 mol��L��1�������Һ��pH���¶ȱ仯��ͼ��
�����з���0.1 mol��L��1NH4Al(SO4)2��pH���¶ȱ仯��������________(����ĸ)��
������ʱ��0.1 mol��L��1NH4Al(SO4)2��2c(SO42-)��c(NH4+)��3c(Al3��)��________mol��L��1(����ֵ����ʽ)��
(3)����ʱ����100 mL 0.1 mol��L��1NH4HSO4��Һ�еμ�0.1 mol��L��1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ2��ʾ���Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶�������____________����b�㣬��Һ�и�����Ũ���ɴ�С������˳����______________��
��.pC��ָ��ϡ��Һ���������ʵ���Ũ�ȵij��ö�����ֵ������pH����ij��Һ���ʵ�Ũ��Ϊ1��10��3mol��L��1�������Һ�и����ʵ�pC����lg10��3��3����֪H2CO3��Һ�д�������ƽ�⣺CO2��H2O![]() H2CO3��H2CO3
H2CO3��H2CO3![]() H����HCO3-��HCO3-
H����HCO3-��HCO3-![]() H����CO32-ͼ3ΪH2CO3��HCO3-��CO32-�ڼ���ǿ���ǿ����Һ�ﵽƽ��ʱ��Һ�����ֳɷֵ�pCpHͼ����ش��������⣺
H����CO32-ͼ3ΪH2CO3��HCO3-��CO32-�ڼ���ǿ���ǿ����Һ�ﵽƽ��ʱ��Һ�����ֳɷֵ�pCpHͼ����ش��������⣺

(1)��pH��9ʱ��H2CO3��Һ��Ũ�����ĺ�̼Ԫ�ص�����Ϊ______��
(2)pH<4ʱ����Һ��H2CO3��pC����Լ����3��ԭ����____________________��
���𰸡�С�� A 10��3��10��11 a c(Na��)>c(SO42-)>c(NH4+)>c(OH��)��c(H��) HCO3- c(H��)�����H2CO3![]() H����HCO3-ƽ�������ƶ����ų�CO2��̼��Ũ�ȱ��ֲ���
H����HCO3-ƽ�������ƶ����ų�CO2��̼��Ũ�ȱ��ֲ���
��������
��.(1) NH4Al(SO4)2��Al3+ˮ�����������NH4+ˮ�⣬HSO4-�����H+ͬ������NH4+ˮ�⣻
(2)��NH4Al(SO4)2ˮ�⣬��Һ�����ԣ������¶���ˮ��̶�����
�ڸ��ݵ���غ㶨�ɽ��⣻
(3) a��b��c��d�ĸ��㣬���ݷ�Ӧ���Ĺ�ϵ��a��ǡ��������H+����Һ��ֻ��(NH4)2SO4��Na2SO4��b��c��d������Һ������NH3H2O��(NH4)2SO4���Դٽ�ˮ�ĵ��룬��NH3H2O����ˮ�ĵ��룮b����Һ�����ԣ�
��.(1) ����pC����֪��pCֵԽ������Ũ��ԽС����֮��pCֵԽС������Ũ��Խ����ͼ������жϣ�
(2) ������Ũ��Խ��Խ����̼����룻�����ж�����̼���ɡ�
��.(1) NH4Al(SO4)2��NH4HSO4�е�NH4+������ˮ�⣬����NH4Al(SO4)2��Al3+ˮ�����������NH4+ˮ�⣬HSO4-�����H+ͬ������NH4+ˮ�⣬��ΪHSO4-�������ɵ�H+Ũ�ȱ�Al3+ˮ�����ɵ�H+Ũ�ȴ�����NH4HSO4��NH4+ˮ��̶ȱ�NH4Al(SO4)2�е�С��
(2)��NH4Al(SO4)2ˮ�⣬��Һ�����ԣ������¶���ˮ��̶�����pH��С�����ϵ�����ΪA��
�ڸ��ݵ���غ㣬�������2c(SO42-)-c(NH4+)-3c(Al3+)=c(H+)-c(OH-)=(10��3��10��11)molL-1��
(3) a��b��c��d�ĸ��㣬���ݷ�Ӧ���Ĺ�ϵ��a��ǡ��������H+����Һ��ֻ��(NH4)2SO4��Na2SO4��b��c��d������Һ������NH3H2O��(NH4)2SO4���Դٽ�ˮ�ĵ��룬��NH3H2O����ˮ�ĵ��룮b����Һ�����ԣ�����Һ����(NH4)2SO4��Na2SO4��NH3H2O���ֳɷ֣�a��ʱc(Na+)=c(SO42-)��b��ʱc(Na+)��c(SO42-)������NԪ����SԪ�صĹ�ϵ�����Եó�c(SO42-)��c(NH4+)����c(Na+)��c(SO42-)��c(NH4+)��c(OH-)=c(H+)��
��.(1) ����pC����֪��pCֵԽ������Ũ��ԽС����֮��pCֵԽС������Ũ��Խ����ͼ��֪��pH=9ʱ��pC��С����HCO3-������HCO3-Ũ�����
(2) pH��4ʱ����Һ�У�c(H+)�����H2CO3H++HCO3-ƽ�������ƶ��ų�CO2������̼��Ũ�Ȳ��䣬̼��Ϊ������Һ��������Һ��H2CO3��pC����Լ����3��

����Ŀ��������ͼ��ʾװ�ý�������ʵ��,�ܵó���Ӧʵ����۵���(����)
ѡ�� | �� | �� | �� | ʵ����� |
|
A | Ũ���� | MnO2 | NaBr��Һ | ������Cl2>Br2 | |
B | Ũ��ˮ | ��ʯ�� | AgNO3��Һ | AgOH�������� | |
C | Ũ���� | Na2SO3 | FeCl3��Һ | SO2���л�ԭ�� | |
D | ϡ���� | Na2CO3 | Na2SiO3��Һ | �ǽ����ԣ�Cl>C>Si |
A.AB.BC.CD.D