��Ŀ����
����Ŀ������������ա�
��.�����£�ijˮ��ҺM�д��ڵ�������Na����A2����HA����H����OH�������ڵķ�����H2O��H2A����������ش��������⣺
(1)д����H2A�ĵ��뷽��ʽ��
________________________________________________________________________��
(2)ʵ����NaHA��Һ��pH>7�������NaHA��Һ�Լ��Ե�ԭ��
________________________________________________________________________��
��д��NaHA��Һ������Ũ���ɴ�С��˳��
________________________________________________________________________��
��.(1)��ͼ�ױ�ʾ����ͬŨ�ȵ�NaOH��Һ�ֱ�ζ�Ũ����ͬ������һԪ�ᣬ��ͼ��ȷ��������ǿ����________(�����١����ڡ������ۡ�)����ͼ�ұ�ʾ����ͬŨ�ȵ�AgNO3����Һ�ֱ�ζ�Ũ����ͬ�ĺ�Cl����Br����I���Ļ����Һ����ͼ��ȷ�����ȳ�����������________��

(2)25 ��ʱ��Ksp(AgCl)��1.8��10��10����1 L 0.1 mol��L��1 NaCl��Һ�м���1 L 0.2 mol��L��1AgNO3��Һ����ַ�Ӧ����Һ��c(Cl��)��________ mol��L��1(�����Ϻ���Һ������仯���Բ���)��
���𰸡�H2A![]() H����HA����HA��
H����HA����HA��![]() H����A2�� HA����ˮ��̶ȴ��������̶� c(Na+)>c(HA-)>c(OH-)>c(H+)>c(A-) �� I�� 3.6��10��9
H����A2�� HA����ˮ��̶ȴ��������̶� c(Na+)>c(HA-)>c(OH-)>c(H+)>c(A-) �� I�� 3.6��10��9
��������
��.��1��������Һ�д��������жϣ���Ԫ����ֲ����룻
��2��0.1mol/LNaHA��Һ��HA-�ĵ���̶ȴ���ˮ��̶ȣ���Һ�����ԣ�H2A��Һ�е�һ����������������������˵ڶ������룬��NaHA��HA-�ĵ���̶ȴ���H2A��HA-�ĵ���̶ȣ�
��.(1) Ũ����ͬ��3��һԪ�ᣬ�۵�pH��С��˵��������ǿ��Ksp(AgI)��С����I�����ȳ�����
(2) NaCl��Һ�м���AgNO3��Һ������ӦNaCl+AgNO3=AgCl��+NaNO3������Ksp(AgCl)��c(Ag+) ��c(Cl��)���㡣
��.��1����Һ�д���H2A��A2-��HA-����H2A��Һ�д��ڵ���ƽ�⣬˵��H2AΪ���ᣬ����뷽��ʽΪ��H2A![]() H����HA����HA��
H����HA����HA��![]() H����A2����
H����A2����
(2) NaHA��Һ�д���HA���ĵ���ƽ�⣨HA��![]() H����A2������ˮ��ƽ��(HA��+H2O
H����A2������ˮ��ƽ��(HA��+H2O![]() OH-��H2A)��ʵ����NaHA��Һ��pH>7��˵��HA����ˮ��̶ȴ��������̶ȣ�
OH-��H2A)��ʵ����NaHA��Һ��pH>7��˵��HA����ˮ��̶ȴ��������̶ȣ�
NaHA��Һ��HA-�ĵ���̶ȴ���ˮ��̶ȣ���Һ�����ԣ�����Һ������Ũ�ȴ�С��ϵΪ��c(Na+)>c(HA-)>c(OH-)>c(H+)>c(A-)��
��.(1) Ũ����ͬ��3��һԪ�ᣬ�۵�pH��С��˵��������ǿ��������ȫ��I��Ũ����С��˵��Ksp(AgI)��С����I�����ȳ�����
(2) NaCl��Һ�м���AgNO3��Һ������ӦNaCl+AgNO3=AgCl��+NaNO3��AgNO3��ʣ�࣬���Һ��c(AgCl)��![]() (0.2mol/L-0.1mol/L)=0.05mol/L��AgCl(s)��ˮ�д����ܽ�ƽ�⣺AgCl(s)
(0.2mol/L-0.1mol/L)=0.05mol/L��AgCl(s)��ˮ�д����ܽ�ƽ�⣺AgCl(s) ![]() Ag+(aq)+Cl-(aq)������Ksp(AgCl)��c(Ag+) ��c(Cl��)����ôc(Cl��)��
Ag+(aq)+Cl-(aq)������Ksp(AgCl)��c(Ag+) ��c(Cl��)����ôc(Cl��)��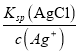 =
=![]() =3.6��10��9mol��L��1��
=3.6��10��9mol��L��1��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��Ϊ�ⶨ��ʽ̼����[COx(OH)y(CO3)z]�Ļ�ѧ��ɣ��о���ѧϰС���ͬѧ�������ͼ��ʾ��װ�ý���ʵ��̽������֪����ʽ̼��������ʱ�ɷֽ��������������
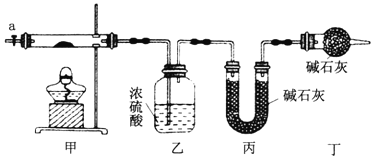
��1������ͼ��ʾװ����װ����������___________����ȡ3.65g��Ʒ����Ӳ�ʲ������ڣ����ȣ�����װ����_______(��ʵ������)��ֹͣ���ȣ�����a������ͨ����������ӣ�ͨ�������Ŀ����__________��
��2��ijͬѧ��Ϊ����ʵ���д���һ��ȱ�ݣ���ȱ����_________��
��3��ͨ����ȷʵ�����ҡ���װ�����طֱ�Ϊ0.36g��0.88g����ü�ʽ̼���ܵĻ�ѧʽΪ__________��
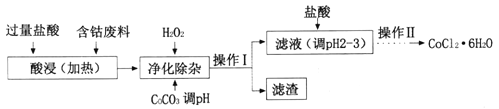
��4��CoCl2��6H2O���������ˮ������Ӽ����Ժ��ܷ���(������Fe��Al������)��ȡCOCl2��6H2O��һ�ֹ������£�
��֪��25��ʱ
������ | Fe(OH)3 | Fe(OH)2 | CO(OH)2 | Al(OH)3 |
��ʼ������pH�� | 2.3 | 7.5 | 7.6 | 3.4 |
��ȫ������pH�� | 4.1 | 9.7 | 9.2 | 5.2 |
�پ�������ʱ������H2O2������Ӧ�����ӷ���ʽΪ______________��
�ڼ���CoCO3��pHΪ5.2��7.6�������I��õ������ɷ�Ϊ_________��
�ۼ��������pHΪ2~3��Ŀ��Ϊ___________��
�ܲ��������Ϊ����Ũ������ȴ�ᾧ�����ˡ�



