��Ŀ����
ij��Һ�к��е����ӿ�����K+��Ba2+��A13+��Mg2+��AlO2����CO32����SiO32����Cl���еļ��֣��ֽ�������ʵ�飺
��ȡ������Һ������������Һ��������������
����ȡ����ԭ��Һ����μ���5mL 0.2mol��L-1���ᣬ�����������ǣ���ʼ���������������࣬���������������������壬��������������ʧ��
���������ڳ�����ʧ�����Һ�У��ټ�����������������Һ�ɵõ�����0.43g��
����˵������ȷ����
| A������Һ��һ������Ba2+��Mg2+��A13+��SiO32����Cl�� |
| B������Һ��һ������K+��AlO2����CO32����Cl�� |
| C������Һ�Ƿ���K+������ɫ��Ӧ������ɫ�ܲ���Ƭ�� |
| D�����ܺ���Cl�� |
B
����

���и���������ˮ��Һ���ܴ����������
| A��Na+��Fe3+��I����CO32�� | B��K+��Fe2+��Cl����NO3�� |
| C��H+��Na +��SiO32����CH3COO�� | D��Ag+��K+��Br����NO3�� |
���и���������ˮ��Һ���ܴ���������ǣ� ��
| A��Na+��HCO3-��SO32-��OH- | B��Al3+��H+��SiO32-��I- |
| C��Fe2+��K+��NO3-��SO42- | D��Fe3+��NH4+��ClO-��CO32- |
��֪SO32-�Ļ�ԭ�Դ���I���Ļ�ԭ�ԣ�ij��ɫ��Һ�п��ܺ���I����NH4+��Cu2����SO32-�������Һ�м���������ˮ����Һ�Գ���ɫ�������й�����Һ��ɵ��ж���ȷ����
�ٿ϶�����I�����ڿ϶�����Cu2�����ۿ϶�����SO32-���ܿ��ܺ���I��
| A���٢� | B���٢ڢ� | C���ۢ� | D���ڢۢ� |
����ɫˮ��Һ���ܴ��������һ�������ǣ� ��
| A��Na����Ag����Br����MnO4- |
| B��K����Al3����AlO2-��SO42- |
| C��Fe3����H����I����SO32- |
| D��Mg2����NH4+��NO3-��Cl�� |
ij��Һ�п��ܺ���Na����NH4+��Ba2����SO42-��I����S2�����ֱ�ȡ��������pH�Ʋ��ԣ���Һ�������ԣ��ڼ���ˮ�͵�������������Ϊȷ������Һ����ɣ���������������
�� ��
| A��Na�� | B��SO42- | C��Ba2�� | D��NH4+ |
�������ӷ���ʽ��ȷ����
| A������ˮ��Ӧ��Na+2H2O=Na+ +2OH- + H2�� |
| B����������Һ�������Һ��ϣ�SiO3 2-+2H+=H2SiO3�� |
| C��0.01mol/L NH4Al(SO4)2 ��Һ��0.02mol/L Ba(OH)2��Һ�������ϣ�NH4+ +Al3++2SO42- +2Ba2+ +4OH- ="2" Ba SO4��+Al��OH��3��+NH3��H2O |
D��Ũ�����м���������۲����ȣ�Fe+3NO3-+6H+ Fe3+ +3NO2+3H2O Fe3+ +3NO2+3H2O |
���и�����������������ԭ��Ӧ�����ܴ����������
| A��H����Fe2����Cr2O72-��SO42- |
| B��Ca2����H����SO42-��HCO3- |
| C��Na����Cu2����Cl����S2�� |
| D��Fe3����K����SCN����Cl�� |
�����й����ӷ�Ӧ�����ӷ���ʽ�������У���ȷ����
| A����ʹpH��ֽ�����ɫ����Һ�У�Fe3����Cl����Ba2����Br���ܴ������� |
B�����Ե缫����Ȼ�����Һ��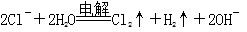 |
C��þ�뼫ϡ���ᷴӦ��������淋����ӷ���ʽΪ4Mg��6H���� =4Mg2���� =4Mg2���� ��3H2O ��3H2O |
| D����10 mL 0.1 mol��L��1 KAl(SO4)2��Һ��10 mL 0.2 mol��L��1Ba(OH)2��Һ��ϣ��õ��ij�����Al(OH)3��BaSO4�����ʵ���֮��Ϊ1��2 |