��Ŀ����
�������ӷ���ʽ��ȷ����
| A������ˮ��Ӧ��Na+2H2O=Na+ +2OH- + H2�� |
| B����������Һ�������Һ��ϣ�SiO3 2-+2H+=H2SiO3�� |
| C��0.01mol/L NH4Al(SO4)2 ��Һ��0.02mol/L Ba(OH)2��Һ�������ϣ�NH4+ +Al3++2SO42- +2Ba2+ +4OH- ="2" Ba SO4��+Al��OH��3��+NH3��H2O |
D��Ũ�����м���������۲����ȣ�Fe+3NO3-+6H+ Fe3+ +3NO2+3H2O Fe3+ +3NO2+3H2O |
C
����

��ϰ��ϵ�д�
 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д� ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д�
�����Ŀ
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����( )
| A������ʯ�����ᷴӦ��CO32��+ 2H+��H2O+CO2�� |
B����NH4HCO3��Һ�мӹ�����NaOH��Һ�����ȣ�NH4++OH��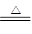 NH3��+H2O NH3��+H2O |
| C��������SO2ͨ���䰱ˮ�У�SO2+NH3��H2O=HSO3��+NH4+ |
| D����ϡ����ϴ���Թ��ڱڵ�������Ag+2H��+NO3��=Ag��+NO��+H2O |
ijpH=1��X��Һ�п��ܺ���Fe2+��A13+��NH4+��CO32�D��SO32�D��SO42�D��C1�D�е������֣���ȡX��Һ��������ʵ�飬ʵ����̼��������£�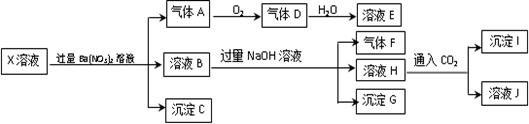
����˵����ȷ����
| A������A��NO2 |
| B��X�п϶�����Fe2+��A13+��NH4+��SO42- |
| C����ҺE������F���ܷ�����ѧ��Ӧ |
| D��X�в���ȷ���������� A13+��C1- |
�±��й������ӷ���ʽ���ۺ�������
| ѡ�� | ��ѧ��Ӧ�������ӷ���ʽ | ���� |
| A | ����������ˮ��CH3COO����H2O===" " CH3COOH��OH�� | ��ȷ |
| B | ������������Һ�еμ������Ȼ����� Al3����3OH��===Al(OH)3�� | ����Al(OH)3�������������� |
| C | �����뵽������ϡ������Һ�У�3Fe �� 8H����2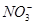 ===3Fe2���� 2NO�� �� 4H2O ===3Fe2���� 2NO�� �� 4H2O | ��ȷ |
| D | ������������Һ�еμ�����������Һ�����������ʵ�����ࣺ3Ba2����6OH����2Al3����3 === 3BaSO4����2Al(OH)3�� === 3BaSO4����2Al(OH)3�� | ����Al3����OH���Ļ�ѧ������֮��Ϊ1��3 |
����ȷ��ʾ���з�Ӧ�����ӷ���ʽ�� �� ��
| A���ù�����ˮ���չ�ҵβ���е�SO2��2NH3��H2O��SO2=2NH4+��SO32-��H2O |
B���Ȼ�����Ũ�����ϼ��ȣ�H2SO4��2Cl�� SO2����Cl2����H2O SO2����Cl2����H2O |
| C����������������ϡ���3Fe2����4H����NO3-=3Fe3����NO����3H2O |
| D��������Һ�е���Ba(OH)2��ҺʹSO42-ǡ����ȫ������2Ba2����3OH����Al3����2SO42-=2BaSO4����Al(OH)3�� |
ij��Һ�к��е����ӿ�����K+��Ba2+��A13+��Mg2+��AlO2����CO32����SiO32����Cl���еļ��֣��ֽ�������ʵ�飺
��ȡ������Һ������������Һ��������������
����ȡ����ԭ��Һ����μ���5mL 0.2mol��L-1���ᣬ�����������ǣ���ʼ���������������࣬���������������������壬��������������ʧ��
���������ڳ�����ʧ�����Һ�У��ټ�����������������Һ�ɵõ�����0.43g��
����˵������ȷ����
| A������Һ��һ������Ba2+��Mg2+��A13+��SiO32����Cl�� |
| B������Һ��һ������K+��AlO2����CO32����Cl�� |
| C������Һ�Ƿ���K+������ɫ��Ӧ������ɫ�ܲ���Ƭ�� |
| D�����ܺ���Cl�� |
���з��ӻ�������ָ���ķ�ɢϵ���ܴ��������һ����
| A��������Һ�� Na+��K+��NO3-��NH3��H2O |
| B�������� C2H2��CO2��SO2��NO |
| C�������������壺 H+��K+��S2-��Br- |
| D�����������Һ�� H+��Na+��SO42-�������Ƿ��� |
ij��Һ�к���NO��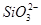 ��
��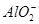 ��S2��4�����ӣ��������м�����������ᣬ�Ȳ����裬�ټ������������������Һ������Һ��������Ŀ�������ٵ���
��S2��4�����ӣ��������м�����������ᣬ�Ȳ����裬�ټ������������������Һ������Һ��������Ŀ�������ٵ���
| A��ֻ��S2�� | B��S2����NO3- |
C��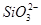 ��S2����NO3- ��S2����NO3- | D��4�����Ӷ����� |
