��Ŀ����
�����й����ӷ�Ӧ�����ӷ���ʽ�������У���ȷ����
| A����ʹpH��ֽ�����ɫ����Һ�У�Fe3����Cl����Ba2����Br���ܴ������� |
B�����Ե缫����Ȼ�����Һ�� |
C��þ�뼫ϡ���ᷴӦ��������淋����ӷ���ʽΪ4Mg��6H���� =4Mg2���� =4Mg2���� ��3H2O ��3H2O |
| D����10 mL 0.1 mol��L��1 KAl(SO4)2��Һ��10 mL 0.2 mol��L��1Ba(OH)2��Һ��ϣ��õ��ij�����Al(OH)3��BaSO4�����ʵ���֮��Ϊ1��2 |
A
����

ijpH=1��X��Һ�п��ܺ���Fe2+��A13+��NH4+��CO32�D��SO32�D��SO42�D��C1�D�е������֣���ȡX��Һ��������ʵ�飬ʵ����̼��������£�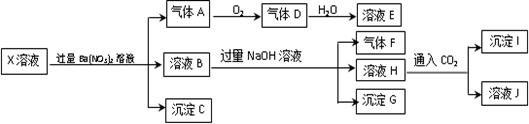
����˵����ȷ����
| A������A��NO2 |
| B��X�п϶�����Fe2+��A13+��NH4+��SO42- |
| C����ҺE������F���ܷ�����ѧ��Ӧ |
| D��X�в���ȷ���������� A13+��C1- |
���и������ӣ��ڼ����������ܴ������棬��ǿ���������·���������ԭ��Ӧ����
| A��Mg2+��Na+��SO42-��HCO3�� | B��Na+��K+��NO3����SO32�� |
| C��K+��Na+��SiO32����ClO�� | D��NH4+��Na+��SO42����NO3�� |
�����£����и���������ָ����Һ��һ���ܴ����������
| A��0��1mol/L KMnO4��Һ��K+��Na+��I����Cl�� |
| B�����ܽ�Al2O3����Һ��Na+��K+��HCO3����NO3�� |
| C������KSCN�Ժ�ɫ����Һ��K+��Mg2+��Cl����SO42�� |
| D��ˮ�����c(OH��)��10��5mol/L����Һ��Na+��Fe2+��Cl����NO3�� |
ij��Һ�к��е����ӿ�����K+��Ba2+��A13+��Mg2+��AlO2����CO32����SiO32����Cl���еļ��֣��ֽ�������ʵ�飺
��ȡ������Һ������������Һ��������������
����ȡ����ԭ��Һ����μ���5mL 0.2mol��L-1���ᣬ�����������ǣ���ʼ���������������࣬���������������������壬��������������ʧ��
���������ڳ�����ʧ�����Һ�У��ټ�����������������Һ�ɵõ�����0.43g��
����˵������ȷ����
| A������Һ��һ������Ba2+��Mg2+��A13+��SiO32����Cl�� |
| B������Һ��һ������K+��AlO2����CO32����Cl�� |
| C������Һ�Ƿ���K+������ɫ��Ӧ������ɫ�ܲ���Ƭ�� |
| D�����ܺ���Cl�� |
�����£����и���������ָ����Һ��һ���ܴ����������
A�����ܽ�Al2O3����Һ��Na����K���� �� �� |
| B��0.1 mol��L��1Ca(ClO)2��Һ��K����Na����I����Cl�� |
C����ʹ�����Ժ�ɫ����Һ��K����Fe2����Cl���� |
D������KSCN�Ժ�ɫ����Һ��Na����Mg2����Cl���� |
������ط�Ӧ�����ӷ���ʽ��д��ȷ����
| A������������������Fe(OH)3��3H��=Fe3����3H2O |
| B������ͭ��Һ�����ԣ�Cu2����2H2O=Cu(OH)2����2H�� |
C����̼�������Һ�мӹ���ʯ��ˮ�����ȣ� ��OH��=NH3����H2O ��OH��=NH3����H2O |
D�����ữ�ĸ��������Һ����˫��ˮ��2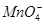 ��6H����5H2O2=2Mn2����5O2����8H2O ��6H����5H2O2=2Mn2����5O2����8H2O |
ij��Һ�к���NO��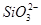 ��
��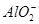 ��S2��4�����ӣ��������м�����������ᣬ�Ȳ����裬�ټ������������������Һ������Һ��������Ŀ�������ٵ���
��S2��4�����ӣ��������м�����������ᣬ�Ȳ����裬�ټ������������������Һ������Һ��������Ŀ�������ٵ���
| A��ֻ��S2�� | B��S2����NO3- |
C��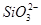 ��S2����NO3- ��S2����NO3- | D��4�����Ӷ����� |
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����
��
| A���ð�ˮ�ܽ��Ȼ���������Ag+��2 NH3��H2O��[Ag(NH3)2]+��2H2O |
| B����������ͨ���Ȼ�����Һ��SO2��2Fe3+��2H2O��SO42����2Fe2+��4H+ |
| C�����������Һ�еμ�����NaOH��Һ��H+��NH4+��2OH����NH3��H2O��H2O |
| D���������������ۻ�ԭNaNO2��NO2����2Al��3OH����H2O��2AlO2����NH3��H2O |