��Ŀ����
����Ŀ��(1)��ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽�������� ______________��
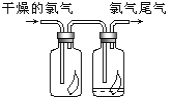
(2)Ϊ��ֹ����β����Ⱦ����������________��Һ���ն����������ԭ����(�û�ѧ����ʽ��ʾ)____________________��
(3)��ҵ�ϳ������۵�ʯ�������չ�ҵ����β���Ƶ�Ư�ۣ�Ư�۵���Ч�ɷ���_______(�ѧʽ),����¶���ڿ����е�Ư�ۣ���ϡ����������������_______(����ĸ������)��
A��O2 B��Cl2 C��CO2 D��HClO
���𰸡��������ɫ��������������ʪ����ɫ������ɫ NaOH Cl2+2NaOH=NaCl+NaClO+H2O Ca(ClO)2 C
��������
���⿼��������Ư���ԣ��������ķ�Ӧ�Լ�Ư��ʧЧ��ԭ�����ش�ʱҪע�����ԵĹ淶���Ͻ���
��1�����������û��Ư�����ã���ʪ����������Ϊ������ˮ��Ӧ�����˴����������Ư�����á����ԣ�����������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽�������ǣ��������ɫ��������������ʪ����ɫ������ɫ��
��2��Ϊ��ֹβ���е�������Ⱦ������ͨ����ŨNaOH��Һ�������գ���Ӧ�Ļ�ѧ����ʽΪ��Cl2+2NaOH=NaCl+NaClO+H2O��
��3����ҵ�ϳ������۵�ʯ�������չ�ҵ����β���Ƶ�Ư�ۣ�2Cl2+2Ca(OH)2=Ca(ClO)2+CaCl2+2H2O��Ư�۵���Ҫ�ɷ���Ca(ClO)2��CaCl2����Ч�ɷ���Ca(ClO)2��Ư�۳���¶���ڿ����л���CO2��ˮ��Ӧ��Ca(ClO)2+CO2+H2O=CaCO3+2HClO�����ù����м�ϡ���������Ӧ��CaCO3+2HCl=CaCl2+H2O+CO2����������������Ҫ��CO2���塣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�