��Ŀ����
10���Ǒz�ᣨH3PO3���Ƕ�Ԫ�ᣬ������NaOH��Һ��Ӧ����Na2HPO3����1��PCl3ˮ�����ȡ�����ᣬ��ѧ����ʽΪPCl3+3H2O�TH3PO3+3HCl��
��2��H3PO3��Һ�д��ڵ���ƽ�⣺H3PO3?H2PO3-+H+��
��ij�¶��£�0.10mol��L-1 �� H3PO3 ��Һ pH=1.6������Һ�� c��H+��=2.5x 10-2 mol��L-1�����¶�����������ƽ���ƽ�ⳣ��K=8.3��10-3��H3PO3�ĵڶ���������Բ��ƣ����������λ��Ч���֣���
�ڸ���H3PO3�����ʿ��Ʋ�Na2HPO3ϡ��Һ��pH��7 �����������=��������������Ũ���ɴ�С��˳����C��Na+����C��HPO32-����C��OH-����C��H2PO3-����C��H+����
��3�����������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽΪH3PO3+I2+H2O�T2HI+H3PO4��
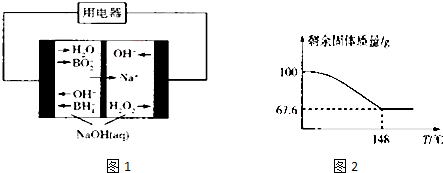
��4�����Na2HPO3��ҺҲ�ɵõ������ᣬװ��ʾ��ͼ��ͼ��

�������ĵ缫��ӦʽΪ2H++2e-�TH2��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪHPO32-+2H+�TH3PO3��
���� ��1��PCl3ˮ�����ȡ�������HCl��
��2���ٵ���ƽ�ⳣ��K=$\frac{C��{H}^{+}����C��{H}_{2}P{{O}_{3}}^{-}��}{C��{H}_{3}P{O}_{3}��}$��
�ڸ���H3PO3������ǿ��ȷ��Na2HPO3��Һ������ԣ�
��3��������͵ⷢ��������ԭ��Ӧ������������ԭ���������������ᣬ�ⱻ��ԭ��������ᣬ�ݴ�д����Ӧ����ʽ��
��4���������ϵõ��ӷ�����ԭ��Ӧ��
�ڲ�Ʒ����HPO32-�������ӽ�� ���������ᣮ
��� �⣺��1��PCl3ˮ�����ȡ����������ᣬˮ�ⷽ��ʽΪ��PCl3+3H2O?H3PO3+3HCl���ʴ�Ϊ��PCl3+3H2O?H3PO3+3HCl��
��2����H3PO3 �TH++H2PO3-
��ʼŨ�� 0.10 0 0
��ӦŨ�� 2.5��10-2 2.5��10-2 2.5��10-2
ƽ��Ũ��0.10-2.5��10-2 2.5��10-2 2.5��10-2
����ƽ�ⳣ��K=$\frac{C��{H}^{+}����C��{H}_{2}P{{O}_{3}}^{-}��}{C��{H}_{3}P{O}_{3}��}$=$\frac{2.5��1{0}^{-2}��2.5��1{0}^{-2}}{0.10-2.5��1{0}^{-2}}$mol/L=8.3��10-3mol/L��
�ʴ�Ϊ8.3��10-3mol/L��
��H3PO3�����ᣬNa2HPO3��ǿ�������Σ�������ˮ��Һ�ʼ��ԣ���pH��7���ʴ�Ϊ������
��3��������͵ⷢ��������ԭ��Ӧ������������ԭ���������������ᣬ�ⱻ��ԭ��������ᣬ��Ӧ����ʽΪ��H3PO3+I2+H2O=2HI+H3PO4��
�ʴ�Ϊ��H3PO3+I2+H2O=2HI+H3PO4��
��4���������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H++2e-=H2�����ʴ�Ϊ��2H++2e-=H2����
�ڲ�Ʒ����HPO32-�������ӽ�����������ᣬ��Ӧ���ӷ���ʽΪHPO32-+2H+=H3PO3���ʴ�Ϊ��HPO32-+2H+=H3PO3��
���� �����漰ˮ�ⷴӦ��������ԭ��Ӧ���缫��Ӧʽ����д��֪ʶ�㣬�缫��Ӧʽ����д���й�ƽ�ⳣ���ļ����Ǹ߿��ȵ㣬Ӧ�ص����գ������ڿ���ѧ���Ի���֪ʶ���ۺ�Ӧ��������

 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�| A�� | ����һ�ֹ��ۻ����� | |
| B�� | �ڼ���ʱ�˻�������Էֽ�ΪPH3��HI | |
| C�� | ���ֻ����ﲻ�ܸ��Ӧ | |
| D�� | �û�������ֻ���й��ۼ� |
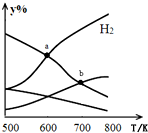 �Զ�����̼������Ϊԭ����ȡ�Ҵ��ķ�ӦΪ��2CO2��g��+6H2��g��$\stackrel{����}{?}$CH3CH2OH��g��+3H2O��g����H��0ijѹǿ�µ��ܱ������У���CO2��H2�����ʵ�����Ϊ1��3Ͷ�ϣ���ͬ�¶���ƽ����ϵ�и����ʵ����ʵ����ٷ�����y%�����¶ȱ仯��ͼ��ʾ������˵����ȷ���ǣ�������
�Զ�����̼������Ϊԭ����ȡ�Ҵ��ķ�ӦΪ��2CO2��g��+6H2��g��$\stackrel{����}{?}$CH3CH2OH��g��+3H2O��g����H��0ijѹǿ�µ��ܱ������У���CO2��H2�����ʵ�����Ϊ1��3Ͷ�ϣ���ͬ�¶���ƽ����ϵ�и����ʵ����ʵ����ٷ�����y%�����¶ȱ仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | a���ƽ�ⳣ��С��b�� | |
| B�� | b�㣬v����CO2��=v����H2O�� | |
| C�� | a�㣬H2��H2O���ʵ������ | |
| D�� | ���������㶨���������H2��v��CO2������ |
| A�� | ��Na2CO3��һ�������³� | B�� | ��NaHCO3�Ķ������³� | ||
| C�� | �Ա���ƽ�� | D�� | ���ж� |
��2��������ͼ���и�װ�����һ��ʵ�飬����֤������Ӧ�������ĸ��ֲ��
| ��� | �� | �� | �� | �� |
| װ�� |  |  |  |  |
��3��ʵ��ʱ�ɹ۲쵽װ�â�Aƿ����Һ��ɫ��Cƿ����Һ����ɫ���Իش�Aƿ��Һ����������֤������������SO2��Bƿ�з�Ӧ�����ӷ���ʽΪ5SO2+2MnO4-+2H2O=5SO42-+2Mn2++4H+��
��4��װ�â������ӵĹ���ҩƷ����ˮ����ͭ������֤�IJ�����ˮ��
��5��װ�â�����ʢ��Һ�dz���ʯ��ˮ����ȷ�������DZ���ǣ�

��֪����ѧ����ʱ��NaClO3�������Լ���ÿ����0.05mol MnO2ʱ����0.02mol NaClO3���������Ϲ������̼���Ϣ���ж�����˵������ȷ���ǣ�������
| A�� | ������е��Լ��ױ�����н�ǿ�Ļ�ԭ�� | |
| B�� | ���������Ӧ�����ӷ���ʽΪ��2ClO3-+5Mn2++4H2O=5MnO2+Cl2��+8H+ | |
| C�� | ���õ�ⷨ����MnO2���������������缫��ӦʽΪMn2+-2e-+2H2O�TMnO2+4H+ | |
| D�� | �ⶨ�����������Һ��Mn2+�ĺ����ɲ����Ƚ�Mn2+ת��ΪMn��Ȼ������0.1 mol•L-1HCl����Һ�ζ��ķ��� |
| A�� | �����������ڹ��������������ȱ��� | |
| B�� | ��ϩ��������Ȼ�̼��Һ��Ӧ����1��2�������� | |
| C�� | ��ϩ��ˮ��Ӧ�����Ҵ� | |
| D�� | ��ϩ�����ۺ����ɾ���ϩ |
| A�� | v��N2��=0.1mol•L-1•s-1 | B�� | v��H2��=0.1mol•L-1•min-1 | ||
| C�� | v��NH3��=0.15mol•L-1•min-1 | D�� | v��H2��=0.3mol•L-1•min-1 |