��Ŀ����
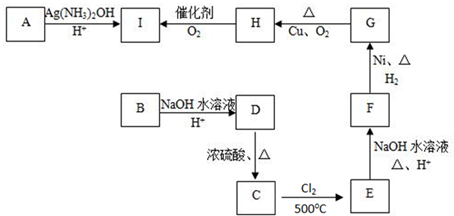
���ʵ�ת����ϵ����ͼ��ʾ(�еķ�Ӧ������ˮ��Һ�н���)������AΪ������������ֵ���ֱ�ӻ��ϵõ�����Ϊ�������ʣ�GΪ�ᣬ����G��Ũ��Һ�з����ۻ���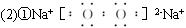
(1)��AΪ���Ṥҵ����Ҫԭ�ϣ�C��ʹƷ����Һ��ɫ��D��ˮ��Һ�м���HNO3�ữ��AgNO3��Һ�а�ɫ�������ɡ���
�ٹ�ҵ�Ϸ�Ӧ������____________����(���豸����)����ҵ�Ϸ�Ӧ����������E���Լ���__________��
��D��ˮ��Һ��__________��(��ᡱ������С�)��
�۷�Ӧ��Ļ�ѧ����ʽ��________________________________________��
(2)����Ϊ����ɫ���壬D��F����Һ���ʼ��ԣ��������������ֱ�պȡA��G��Ũ��Һ��ʹ���ǽӽ����д����������ɡ���
�ټĵ���ʽ��__________��
����
_____________________________________________________________________��
(1)�ٽӴ��� 98.3%������(��Ũ����)
����
��4FeS2+11O2![]() 2Fe2O3+8SO2
2Fe2O3+8SO2
![]()
��4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g)����H=-906.8 kJ��mol-1(���������𰸾���)
4NO(g)+6H2O(g)����H=-906.8 kJ��mol-1(���������𰸾���)
������(1)��AΪ���Ṥҵ��ԭ�ϣ�����O2��Ӧ������SO2����AΪFeS2��BΪFe2O3��D��ˮ��Һ�к���Cl-�����Լ�ΪHCl��DΪFeCl3����Ϊ����Fe.(2)A��G��Ũ��Һ�������ɰ��̣�˵��AΪNH3��GΪHNO3������Na2O2������ŨHNO3�жۻ�������ΪAl��F��NaAlO2.4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(l)����H=-906.8 kJ��mol-1.
4NO(g)+6H2O(l)����H=-906.8 kJ��mol-1.


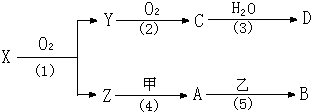
 ��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ����ͼ�����ֲ���δ�г�����A��һ�ֽ������ʣ�D��һ�ַǽ������嵥�ʣ�
��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ����ͼ�����ֲ���δ�г�����A��һ�ֽ������ʣ�D��һ�ַǽ������嵥�ʣ�