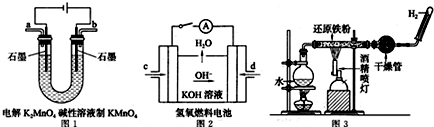
��Ŀ����
8����Ҫ��ش�����������1���û�ѧ����ʽ���ͣ�
����PH�Ʋ�ij�������ȣ���ʼһ��ʱ��������ǿ��2H2SO3+O2�T2H2SO4��
��ij��ɫ�����ڿ�����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��4Fe��OH��+O2+2H2O=4Fe��OH��3��
��2������ػ�ѧ������ͣ�����ͨ����з�̪��ˮ�У���Һ���ɫ��NH3+H2O?NH3•H2O?NH4++OH-��
��3�������ӷ���ʽ��������ԭ����
��ij��ɫ��ĩ����ɫҺ�干�ȣ��л���ɫ�������4H++2Cl-+MnO2$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O��
��װ����������Һ���Լ�ƿ�����ò�����2OH-+SiO2=H2O+SiO32-��
��4��ijNH4Cl�����п��ܻ���KCl���ʣ������һ�����ʵ�飬֤���þ��岻�����ʣ���д����Ҫ�IJ������衢����ͽ��ۣ�
���� ��1���ٿ�ʼʱ��Һ�е������ᱻ�������������������ᣬ����������ǿ��
�ڸ÷�ӦΪ�����������ڿ����б�����������������
��2����ˮ��Һ�ʼ��ԣ���ɫ��̪��Һ������ɫ��
��3���ٸ÷�ӦΪŨ������������̼��ȷ�Ӧ��ȡ������
��ƿ���к��ж������裬��������������������Һ��Ӧ���ɹ����ƺ�ˮ��
��4���Ȼ�識������ֽ⣬���ԶԻ������Ⱥ�������û�й���ʣ�࣬���Ȼ������ʣ�
��� �⣺��1���������������ᱻ�������������ᣬ������Һ������ǿ����Ӧ�Ļ�ѧ����ʽΪ��2H2SO3+O2�T2H2SO4��
�ʴ�Ϊ��2H2SO3+O2�T2H2SO4��
��ij��ɫ�����ڿ�����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ���÷�ӦΪ���������������������������Ĺ��̣���Ӧ����ʽΪ��4Fe��OH��+O2+2H2O=4Fe��OH��3��
�ʴ�Ϊ��4Fe��OH��+O2+2H2O=4Fe��OH��3��
��2��������ˮ��Ӧ����һˮ�ϰ���һˮ�ϰ���������ʣ���������������Ӷ�ʹ��Һ�ʼ��ԣ���ɫ��̪������ɫ�������ӷ���ʽΪ��NH3+H2O?NH3•H2O?NH4++OH-��
�ʴ�Ϊ��NH3+H2O?NH3•H2O?NH4++OH-��
��3����ij��ɫ��ĩ����ɫҺ�干�ȣ��л���ɫ����������û���ɫ����Ϊ��������Ӧ�����ӷ���ʽΪ��4H++2Cl-+MnO2$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O��
�ʴ�Ϊ��4H++2Cl-+MnO2$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O��
�����������벣�����еĶ������跴Ӧ����ճ�����ʹ����ƣ���Ӧ�����ӷ���ʽΪ��2OH-+SiO2=H2O+SiO32-��
�ʴ�Ϊ��2OH-+SiO2=H2O+SiO32-��
��4���Ȼ�識����ֽ⣬���Ȼ��رȽ��ȶ����ݴ˿�֤���þ��岻�����ʣ���������Ϊ��ȡ�����þ�����ྻ������Թ��У����Թܼмӳ��ھƾ����ϳ�ּ��ȣ�һ������Թܵײ������������þ����в������ʣ�
�ʴ�Ϊ��ȡ�����þ�����ྻ������Թ��У����Թܼмӳ��ھƾ����ϳ�ּ��ȣ�һ������Թܵײ������������þ����в������ʣ�
���� ���⿼�������ӷ���ʽ����ѧ����ʽ������ʵ�鷽������Ƶ�֪ʶ����Ŀ�Ѷ��еȣ�ע���������ӷ���ʽ����ѧ����ʽ��дԭ������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ���ѧ�����Ӧ�û���֪ʶ����������ѧʵ��������

 ��У����ϵ�д�
��У����ϵ�д�| A�� | HCl��NaOH��Ӧ���к��ȡ�H=-57.3 kJ/mol����H2SO4��Ca��OH��2��Ӧ���к��ȡ�H=2����-57.3��kJ/mol | |
| B�� | CO��g����ȼ������283.0 kJ/mol����2CO2��g��=2CO��g��+O2��g����Ӧ�ġ�H=+566.0 kJ/mol | |
| C�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ | |
| D�� | Ba��OH��2•8H2O��NH4Cl��Ӧ���ʱ�С��0�������ڳ��������Է����� |
| A�� | �Ȼ���ĵ���ʽΪ H+[${\;}_{•}^{•}$$\underset{\stackrel{••}{CI}}{••}$${\;}_{•}^{•}$]- | |
| B�� | ��Ȳ�Ľṹ��ʽ��ɾ�����ԣ�д�� CHCH | |
| C�� | þ��ԭ�ӽṹʾ��ͼ | |
| D�� | ̼����������ˮ���뷽��ʽ��NaHCO3�TNa++H++CO32- |
| Ԫ������ | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶/10-10m | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 1.43 |
| ����ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | +3 | |
| ��ͻ��ϼ� | -2 | -3 | -1 | -3 |
| A�� | Ԫ�آ����Ľ�������ȣ�ǰ��ǿ�ں��� | |
| B�� | ����8��Ԫ���У�Ԫ�آݵ�����������Ӧ��ˮ����������ǿ | |
| C�� | Ԫ�آߵ���̬�⻯����Ԫ�آܵ���̬�⻯����Ƚϣ�ǰ���ȶ���ǿ�����߷е�� | |
| D�� | Ԫ�آٷֱ���Ԫ�آں͢��γɵĻ�����������ѧ�����Ͳ�һ����ȫ��ͬ |
| A��̽����ͬ������ͬһ��Ӧ���ʵ�Ӱ�� | B��̽���¶ȶԻ�ѧƽ���Ӱ�� |
 �Լ���������Һ����ˮ�� ��Һ��2mol/L H2SO4��Һ ����ͼ�����Թ���Һ��ɫһ��ʱ�������ɫ�����Թ���Һ��ɫѸ�ٱ���ɫ |  2NO2��g��?N2O4��H��0 �Լ�����ƿ�и������������NO2 ����һ��ʱ����ұ���ƿ��������ɫ��dz�������ƿ��������ɫ���� |
| C��̽�����ᡢ̼�ᡢ���������ǿ�� | D����֤��ӵ��������������� |
 �Լ���0.1mol/L������Һ���������� ��Һ0.1mol/L Na2CO3��Һ ����ͼ�����Թ���Һ��������ݣ����Թ����������� | 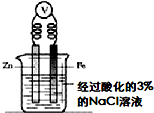 �Լ����ữ��3%��NaCl��Һ�� ���軯����Һ ����һ��ʱ������ձ��еμ�2�����軯����Һ����������ɫ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| C | ||
| A | R | B |
| D |
| A�� | 4Z | B�� | 4Z+10 | C�� | 4Z+14 | D�� | 4Z+5 |
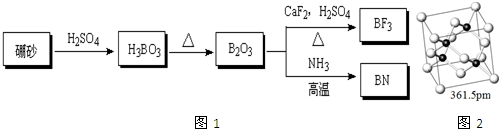
 ��BN��BԪ�صĻ��ϼ�Ϊ+3��
��BN��BԪ�صĻ��ϼ�Ϊ+3�� ������ȴ�����4�֣�
������ȴ�����4�֣�