��Ŀ����
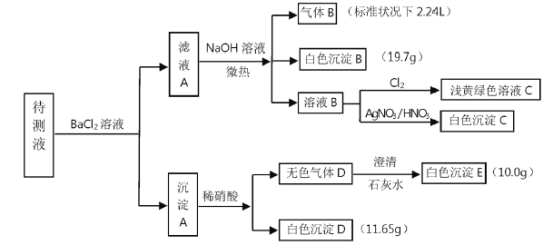
����Ŀ����֪1L��ɫ����Һ�г�����0.2mo/L��Na���⣬�����ܺ����������е�һ�ֻ��֣�
������ | K+��NH4+��Ca2+��Ba2+��Fe3+ |
������ | Cl-��Br-��CO32-��HCO3-��SO42- |
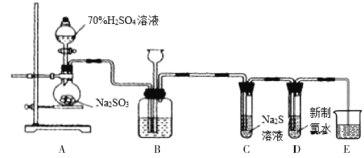
�ֽ�����ͼʵ�����(ÿ��ʵ�������Լ�������)
(1)������B��ȷ������Һ�к��е�������___________��
(2)�ɰ�ɫ����D�Ͱ�ɫ����E�����ж�����Һ��һ�����е�������___________���ݴ˿���ȷ����Һ��һ�������ڵ�������___________��
(3)�ɰ�ɫ����B��ȷ������Һ�к��е�������___________��
(4)ijͬѧ��Ϊ��Һ��һ�����������ӣ��жϵ�������______________________��
(5)���Ϸ���������Һ��K������СŨ��Ϊ___________��
���𰸡�NH4+ CO32-��SO42- Ca2+��Ba2+ HCO3- ����ҺB��ͨ��������Һ��dz����ɫ���������������Һ�����ְ�ɫ������ 0.1mol/L
��������
��ɫ����Һһ��������Fe3+������Һ���Ȼ�����Һ��Ӧ�õ�����A������Һ�п��ܺ���CO32-��SO42-��������м���ϡ�����������壬���в��ֳ������ܽ⣬����Һ�д���CO32-��SO42-���������ӹ���֪����Һ�в�����Ca2+��Ba2+����ҺA����Ba2+�����������NaOH��Һ�õ�����B����ɫ����B������Һ��һ������NH4+��HCO3-������BΪNH3����ɫ����BΪBaCO3����ҺB��ͨ����������dz����ɫ��Һ����Һ��һ��û��Br-����ҺB�м�����������������Һ�õ���ɫ����C��CΪAgCl��˵����ҺB�к���Cl-�����ڼ����Ȼ�����Һ������ȷ��ԭ��Һ���Ƿ���Cl-���Դ˽����⡣
��1�������Ϸ�����֪����BΪNH3������Һ��һ������NH4+��
(2) ����ɫ����Dͨ��ʯ��ˮ�У�ʯ��ˮ����ǣ���DΪCO2��EΪCaCO3����Һ�к���CO32-�������Ȼ������ɰ�ɫ����������D������ϡ���ᣬDһ����BaSO4�������ж�����Һ��һ�����е�������SO42-���������ӹ��棬һ��������Ca2+��Ba2+��
(3) ��ҺA����Ba2+��˵��A��һ��û��CO32-�����������NaOH��Һ�õ���ɫ����B������Bһ����̼�ᱵ����ԭ����Һ�к��е�������HCO3-��
(4) ��ҺB��ͨ����������dz����ɫ��Һ����Һ��һ��û��Br-��
(5) n(CO32-)=![]() ��n(SO42-)=
��n(SO42-)=![]() ��n(HCO3-)=
��n(HCO3-)=![]() ��n(NH4+)=
��n(NH4+)=![]() ����Һ�е�ɳ����ԣ�2��n(SO42-)+1��n(HCO3-)+2��n(CO32-)=2��0.05mol+1��0.1mol+2��0.1mol=0.4mol��n(NH4+)+n(Na+)=0.1mol��1+0.2mol=0.3mol���������������������������С�ڸ��������������һ������������K������ԭ��Һ�в�����Cl-����K+�����ʵ�����0.4mol��0.3mol=0.1mol��K+Ũ����c(K+)=0.1mol��1L=0.1mol/L��������Cl-����K+��Ũ��Ӧ�ô���0.1mol/L��
����Һ�е�ɳ����ԣ�2��n(SO42-)+1��n(HCO3-)+2��n(CO32-)=2��0.05mol+1��0.1mol+2��0.1mol=0.4mol��n(NH4+)+n(Na+)=0.1mol��1+0.2mol=0.3mol���������������������������С�ڸ��������������һ������������K������ԭ��Һ�в�����Cl-����K+�����ʵ�����0.4mol��0.3mol=0.1mol��K+Ũ����c(K+)=0.1mol��1L=0.1mol/L��������Cl-����K+��Ũ��Ӧ�ô���0.1mol/L��

 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�����Ŀ��ú̿ȼ�չ����л��ͷų�������SO2,�����ƻ���̬����������һ����������������Ԫ����CaSO4����ʽ�̶�,�Ӷ�����SO2���ŷš�����ú̿ȼ�չ����в�����CO�ֻ���CaSO4������ѧ��Ӧ,��������Ч�ʡ���ط�Ӧ���Ȼ�ѧ����ʽ����:
CaSO4(s)+CO(g) ![]() CaO(s)+SO2(g)+CO2(g) ��H1=+218.4kJ��mol-1(��Ӧ��)
CaO(s)+SO2(g)+CO2(g) ��H1=+218.4kJ��mol-1(��Ӧ��)
CaSO4(s)+4CO(g) ![]() CaS(s)+4CO2(g) ��H2= -175.6kJ��mol-1(��Ӧ��)
CaS(s)+4CO2(g) ��H2= -175.6kJ��mol-1(��Ӧ��)
��ش���������:
(1)����ij�¶���,��Ӧ�������(v1)���ڷ�Ӧ�������(v2),�����з�Ӧ���������仯ʾ��ͼ��ȷ����__________��
A.  B.
B. 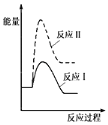
C. 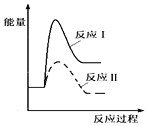 D.
D.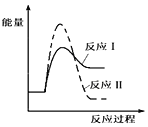
(2)���¶ȡ��ݻ���ͬ�Ҳ����3���ܱ�������,����ͬ��ʽͶ�뷴Ӧ��,���ֺ��¡�����,��÷�Ӧ�ﵽƽ��ʱ���й��������±�(��֪2SO2(g)+O2(g) ![]() 2SO3(g)��H=196.6kJ��mol-1) ��
2SO3(g)��H=196.6kJ��mol-1) ��
���� | �� | �� | �� | (��>,=,<) A.2c1_____c3 B.a+b_____196.6 C.2p2____p3 D.��1+��3___1 |
��Ӧ��Ͷ���� | 2mol SO2��1mol O2 | 2mol SO3 | 4mol SO3 | |
SO3��Ũ�� (mol��L-1) | C1 | C2 | C3 | |
��Ӧ�������仯 | �ų�a kJ | ����b kJ | ����c kJ | |
��ϵѹǿ | P1 | P2 | P3 | |
��Ӧ��ת���� | ��1 | ��2 | ��3 |
(3)���������η������������е�SO2������������,������ͨ�백ˮ��,�����ҺpH�뺬��������ʵ��������ı仯��ϵ��ͼ��ʾ��
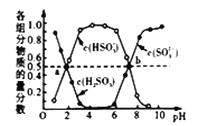
��д��a��ʱn(HSO3-):n(H2SO3)=______��b��ʱ��ҺpH=7,��n(NH4+):n(HSO3-)=_____��
(4)��������ȥ��NO,һ��������,��NH3����NO��Ⱦ,�䷴Ӧԭ��Ϊ4NH3+6NO ![]() 5N2+ 6H2O����ͬ�¶�������,n(NH3):n(NO)�����ʵ���֮�ȷֱ�Ϊ4:l��3:l��1:3ʱ,�õ�NO�ѳ���������ͼ��ʾ��
5N2+ 6H2O����ͬ�¶�������,n(NH3):n(NO)�����ʵ���֮�ȷֱ�Ϊ4:l��3:l��1:3ʱ,�õ�NO�ѳ���������ͼ��ʾ��
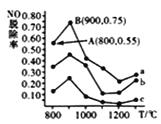
������c��ӦNH3��NO�����ʵ���֮����__________��
������a��NO����ʼŨ��Ϊ6��10-4mg/m3,��A�㵽B�㾭��0.8s,��ʱ�����NO���ѳ�����Ϊ__________mg/(m3��s)��
(5)��֪Ksp(BaSO4)=1��10-10,Ksp(BaCO3)=2.5��10-9,��0.4mol/L Na2SO4����Һ�м�������BaCO3��ĩ(��������仯),��ֽ���,������ӦSO42- (aq)+BaCO3(s) ![]() BaSO4 (s)+CO32-(aq) ���ú����ת���ﵽƽ�⡣��ʱ��Һ�е�c(SO42-)=____mol��L-1(����С�������λ).
BaSO4 (s)+CO32-(aq) ���ú����ת���ﵽƽ�⡣��ʱ��Һ�е�c(SO42-)=____mol��L-1(����С�������λ).