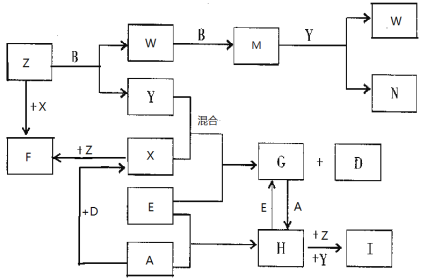
��Ŀ����
����Ŀ����1����298K��100kPaʱ.CH4��ȼ������890.0kJ/mol��д���÷�Ӧ���Ȼ�ѧ����ʽ__________������CH4��CO�Ļ������0.75mol����ȫȼ��������CO2�����18gҺ̬ˮ�����ų�QkJ����(�ٶ�����δ��ʧ)����CH4��CO�����ʵ����ı�Ϊ__________��
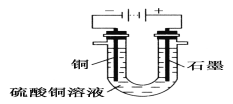
��2�����ü����ȼ����Ӧ���һ��ȼ�������������������Һ���������Һ.���ʯī���缫���ڵ缫�Ϸֱ�ͨ������������ͨ���������ĵ缫�Ϸ����ĵ缫��Ӧ��___________������·��ת��12mol����ʱ��ʵ���ṩ�ĵ�����890.0kJ����õ�ص�����ת��Ч����__________��
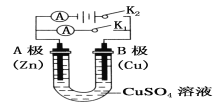
��3����������ʯī������ʢ�б���NaCl��Һ��U�ι����γ���ͼװ�ã�

��������K1�պϣ����������绯ѧ��ʴ�е�__________��ʴ��
��������K2�պϣ����ܷ�Ӧ�����ӷ���ʽΪ__________��
���𰸡���1��CH4(g)+2O2(g)=CO2(g)+2H2O��H=-890.0kJ/mol��2:1
��2��CH4-8e-+10OH-=CO32-+7H2O��2/3(��66.7%)
��3������������2C1-+2H2O![]() 2OH-+H2��+C12��
2OH-+H2��+C12��
��������
�����������1����298Kʱ��1mol CH4����������ȫȼ�����ɶ�����̼��Һ̬ˮ���ų�����890.0kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ��CH4(g)+2O2(g)�TCO2(g)+2H2O(l)��H=-890.0kJ/mol��18��Һ̬ˮ�����ʵ���=![]() =1mol������Hԭ���غ��֪n(CH4)=
=1mol������Hԭ���غ��֪n(CH4)=![]() =0.5mol����n(CO)=0.75mol-0.5mol=0.25mol�����ʵ���֮��Ϊ2:1���ʴ�Ϊ��CH4(g)+2O2(g)�TCO2(g)+2H2O(l)��H=-890.0kJ/mol��2:1��
=0.5mol����n(CO)=0.75mol-0.5mol=0.25mol�����ʵ���֮��Ϊ2:1���ʴ�Ϊ��CH4(g)+2O2(g)�TCO2(g)+2H2O(l)��H=-890.0kJ/mol��2:1��
��2������ʧ���ӷ���������Ӧ������ͨ���������ĵ缫ӦΪ�����������ڸ���ʧȥ���ӣ���������������̼�����ˮ���缫��ӦʽΪ��CH4-8e-+10OH-�TCO32-+7H2O������·��ת��12mol����ʱ��ʵ���ṩ�ĵ�����890.0kJ����õ�ص�����ת��Ч��Ϊ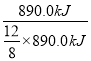 =
=![]() =66.7%���ʴ�Ϊ��CH4-8e-+10OH-�TCO32-+7H2O��66.7%��
=66.7%���ʴ�Ϊ��CH4-8e-+10OH-�TCO32-+7H2O��66.7%��
��3����������K1�պϣ�����ԭ���װ�ã����������绯ѧ��ʴ�е�������ʴ���ʴ�Ϊ��������
��������K2�պϣ����ɵ��أ���Ϊ���������ǵ缫����ʳ��ˮ���ܷ�Ӧ�����ӷ���ʽΪ2C1-+2H2O![]() 2OH-+H2��+C12�����ʴ�Ϊ��2C1-+2H2O
2OH-+H2��+C12�����ʴ�Ϊ��2C1-+2H2O![]() 2OH-+H2��+C12����
2OH-+H2��+C12����