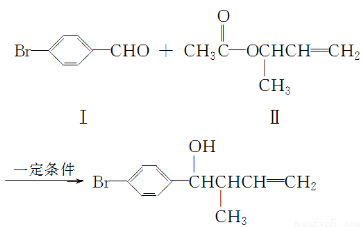
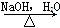��Ŀ����
�����г������ؽ�����Ⱦ���У�����Ǧ���̡������ӡ�������ҵ��ˮ�к��е�Cr2O72-��CrO42-�����õķ��������֡�
����1����ԭ������
�÷��Ĺ�������Ϊ ��
��
���е���������ƽ��2CrO42-����ɫ����2H�� Cr2O72-����ɫ����H2O��
Cr2O72-����ɫ����H2O��
��1��д����������Ӧ��ƽ�ⳣ������ʽ_________________________________��
��2�����ڵ�������Ӧ������˵����ȷ����________��
A��ͨ���ⶨ��Һ��pH�����жϷ�Ӧ�Ƿ��Ѵ�ƽ��״̬
B���÷�ӦΪ������ԭ��Ӧ
C��ǿ���Ի�������Һ����ɫΪ��ɫ
��3��������������ԭ0.1 mol Cr2O72-����Ҫ________mol��FeSO4��7H2O��
��4��������������Cr��OH��3�������������ɵij���Ϊ________������Һ�д������³����ܽ�ƽ�⣺Cr��OH��3��s�� Cr3����aq����3OH����aq������������Cr��OH��3���ܶȻ�Ksp��10��32����c��Cr3��������10��5 mol��L��1ʱ����Ϊc��Cr3�����Ѿ���ȫ�������ֽ���������Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ����д��������̣���____________________________________________________________________________��
Cr3����aq����3OH����aq������������Cr��OH��3���ܶȻ�Ksp��10��32����c��Cr3��������10��5 mol��L��1ʱ����Ϊc��Cr3�����Ѿ���ȫ�������ֽ���������Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ����д��������̣���____________________________________________________________________________��
����2����ⷨ
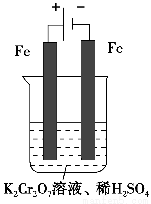
��5��ʵ����������ͼװ��ģ���ⷨ������Cr2O72-�ķ�ˮ�����ʱ������ӦʽΪ________��������ӦʽΪ________���õ��Ľ������������������ɳ�����ȫ����ˮ�ĵ���ƽ��ǶȽ�����ԭ����___________________________________________________________��
��1��K��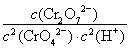
��2��AC
��3��0.6
��4��Fe��OH��3����pH����4ʱ��c��OH������10��10 mol��L��1��c��Cr3������10��32/c3��OH������10��2 mol��L��1��10��5 mol��L��1�����Cr3��û�г�����ȫ
��5��Fe��2e��=Fe2����2H����2e��=H2�����������ɵĽ����������������ƶ���������Ӧ������H����������ˮ�ĵ���ƽ�����ٽ���ˮ�ĵ�����ʹ��Һ��OH����Ũ����������Һ�ʼ���
����������1��ϡ��Һ��H2O��Ũ�ȿ���Ϊ�������ʵ�������Ӧ��ƽ�ⳣ������ʽΪK��c��Cr2O72-��/[c2��CrO42-����c2��H����]����2���ɷ�Ӧ����֪��ƽ�ⷢ���ƶ�����Һ��pH�����仯����pH����ʱ˵����Ӧ�ﵽƽ����A����ȷ��CrO42-��Cr2O72-��Cr��Ϊ��6�����÷�Ӧ����������ԭ��Ӧ��B����������Ի���������Һ��c��Cr2O72-���ϴ�����Һ�ʳ�ɫ��C����ȷ����3���ڵ�������Ӧ��Cr2O72-����ԭΪCr3����0.1 mol Cr2O72-����ԭʱת�Ƶ��ӵ����ʵ���Ϊ0.1 mol��2����6��3����0.6 mol������ԭ��Fe2��������ΪFe3��������Ҫ����0.6 mol FeSO4��7H2O����4���ڵ�������Ӧ��Fe2��������ΪFe3�����ʵ���������Fe��OH��3���ɡ���5�����ص���������������Ӧ��Fe����������Fe�缫��������������������ӦʽΪFe��2e��=Fe2�����������ǵ������Һ�е�H���õ�����������ԭ��Ӧ������������������ӦʽΪ2H����2e��=H2�����������ɵĽ���������������������������Ӧ������H����������ˮ�ĵ���ƽ�����ٽ���ˮ�ĵ�����ʹ��Һ�е�OH��Ũ����������Һ�ʼ������Ӷ�ʹ�������������������γɳ�����

���Ϊ1 L��ij��Һ�к��е����������ʾ��
���� | Cu2�� | Al3�� | NO3- | Cl�� |
| 1 | 1 | a | 1 |
��Pt�缫������Һ������·����3 mol����ͨ��ʱ�����Ե��ʱ��Һ����ı仯����������ܴ��ڵ��ܽ�����������˵����ȷ���� ����������
A��������Һ��pH��0
B��a��3
C����������1.5 mol Cl2
D�����������Ľ�����ͭ����
��һ���������İ�������粒����������Ƶ��ܱ���������У��������������������������������Բ��ƣ����ں㶨�¶���ʹ��ﵽ�ֽ�ƽ�⣺NH2COONH4��s��  2NH3��g����CO2��g��
2NH3��g����CO2��g��
ʵ���ò�ͬ�¶��µ�ƽ�����������±���
�¶�/�� | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 |
ƽ����ѹ ǿ/kPa | 5.7 | 8.3 | 12.0 | 17.1 | 24.0 |
ƽ��������Ũ��/mol�� L��1 | 2.4�� 10��3 | 3.4�� 10��3 | 4.8�� 10��3 | 6.8�� 10��3 | 9.4�� 10��3 |
��1�������жϸ÷ֽⷴӦ�Ѿ��ﵽƽ�����________��
A��2v��NH3����v��CO2��
B���ܱ���������ѹǿ����
C���ܱ������л��������ܶȲ���
D���ܱ������а����������������
��2�����ݱ�����������ʽ����25.0 ��ʱ�ķֽⷴӦƽ�ⳣ����_______________��
��3��ȡһ�����İ�������粒������һ�����������ܱ��������������25.0 ���´ﵽ�ֽ�ƽ�⡣���ں�����ѹ�������������������粒����������________������������������������������������