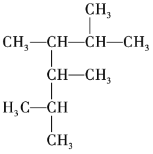
��Ŀ����
����Ŀ���� NA Ϊ�����ӵ�������ֵ������˵����ȷ����
A.��Ӧ 5NH4NO3=2HNO3+4N2��+9H2O������22.4L N2 ʱת�Ƶĵ�����Ϊ3.75NA
B.��״���¼���������Ļ�����干22.4L����ȫȼ�պ�����ʵķ�������һ��ΪNA
C.��1mol Cl2ͨ��ˮ�У��� N(HClO)+N(Cl-)+N(ClO-)=2NA(N��ʾ������)
D.10g��D2O�к��е����������������ֱ�Ϊ5NA ��4NA
���𰸡�B
��������
A. ȱ��������������ܼ���N2�����ʵ��������Ҳ���ܼ��㷴Ӧ�����е���ת����Ŀ��A����
B. ��״����22.4L����������Ļ����������ʵ�����1mol�����ݰ���٤�����ɿ�֪�������к��еķ�����ĿΪNA������ӦCH4+2O2![]() CO2+2H2O�Ƿ�Ӧǰ�������������ķ�Ӧ���ʻ��������ȫȼ�պ����ʵ��ܷ�����һ��ΪNA��B��ȷ��
CO2+2H2O�Ƿ�Ӧǰ�������������ķ�Ӧ���ʻ��������ȫȼ�պ����ʵ��ܷ�����һ��ΪNA��B��ȷ��
C. Cl2��ˮ�ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫת��Ϊ���������N(HClO)+N(Cl-)+N(ClO-)<2NA��C����
D. D2O�����ʵ�����n(D2O)=10g��20g/mol=0.5mol��D2O�����к���10�����ӣ�����10�����ӣ�����0.5mol D2O�к��е�������������������5NA ��D����
�ʺ���ѡ����B��