��Ŀ����
����Ŀ��ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о�,�ܽ��������������H2�ķ�Ӧ:��Zn+����;��Na+ˮ;��Al+NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2,�������������ͼ��ʾ��װ��ͼ:��ش���������:
![]()
��1��д��Al��NaOH��Һ��Ӧ�����ӷ���ʽ_______________________________��
��2���ڵ�ȼH2֮ǰ�����Ƚ���____________________________________________��

��3��ʵ��С���ڵ�ȼ������װ���Ƶõ�H2ʱ,�٢�ʵ���óɹ�,��ȴʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫��,Na��������̫�١����������������Ƶ�����,�ɽ�ʦ˵̫Σ��,����Ϊ����Σ�յ�ԭ����___________________________��
��4��ʵ��С������ơ���(һ�ֲ�����ˮ��Һ̬�л���)��ˮ���ܶȷֱ�Ϊ0.97 g��mL-1��0.88 g��mL-1��1.00 g��mL-1,���ݴ˶�ʵ������˸Ľ����ڸĽ����ʵ����H2����������____________��(���������������ӿ���)
��5����4.6gNa��8.1gAlͶ�뵽������ˮ�У�������Һ�����Ϊ200mL�������ɱ�״���µ�H2�������___________������Һ�����ʵ����ʵ���Ũ����__________________��
���𰸡�.2Al+2H2O+2OH��====2AlO2��+3H2�� �鴿 �϶������ˮ��Ӧ�ų���������,ʹ�Թ���H2��O2�Ļ������ȼ����ը ���� 8.96 L 1 mol��L-1
��������
��1��Al��NaOH��Һ��Ӧ����ƫ�����ƺ���������Ӧ�����ӷ���ʽΪ��2Al+2H2O+2OH��=2AlO2��+3H2������2��H2�ǿ�ȼ�����壬��ȼ�������ڵ�ȼǰ������м촿����3�����Թ��в�Ҫ�������Na������϶������ˮ��Ӧ�ų��������ȣ�ʹ�Թ���H2��O2�Ļ������ȼ����ը����4���ƿ����ḽ�ű������ԸĽ����ʵ����H2���������ʼ�����
��5��4.6gNa�����ʵ���Ϊ![]() =0.2mol��8.1gAl�����ʵ���Ϊ
=0.2mol��8.1gAl�����ʵ���Ϊ![]() =0.3mol��
=0.3mol��
����Na��ˮ��Ӧ��2Na + 2H2O = 2NaOH + H2��
0.2mol 0.2mol 0.1mol
����n��NaOH��=0.2mol��
����NaOH��Al��Ӧ��2Al + 2NaOH + 2H2O = 2NaAlO2 + 3H2��
0.3mol(����) 0.2mol 0.2mol 0.3mol
Al������NaOH��ȫ��Ӧ����Ӧ�������Ϊ0.2molNaAlO2�����ɵ�H2�����ʵ���Ϊ0.1mol+0.2mol=0.3mol����״���µ����Ϊ0.3mol��22.4L/mol=8.96L����Һ������NaAlO2�����ʵ���Ũ����![]() =1mol/L��
=1mol/L��

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�����Ŀ���±������������г��������ʣ������г������ǵ�(��Ҫ���ɷ֡�
��� | �� | �� | �� | �� | �� | �� | �� |
���� | �ƾ� | ���� | ��� | ʳ�� | ͭ���� | �������� | �մ� |
��Ҫ�ɷ� | CH3CH2OH | CH3COOH | NaOH | NaCl | Cu | SO2 | Na2CO3 |
��1������Ա��Т����ߵ���Ҫ�ɷֽ��з���(���ţ������ڵ���ʵ���______�����ڷǵ����_______��
��2�������ڵ�ˮ��Һ��߷�Ӧ�����ӷ���ʽ______________________��
��3��ijͬѧ�âݺ�Ũ���Ṳ�����Ʊ��ޣ���ѧ����ʽΪ��Cu+2H2SO4(Ũ��![]() CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
�����õ����ű������ת�Ƶ����____________��
��ŨH2SO4���ֳ����������ǣ�_______��������ת��0.1molʱ�����������������ʵ���Ϊ_______��
��4����ͼ��ʾijͬѧ����480mL 0.5mol/L ��NaOH��Һ�IJ��ֲ���ʾ��ͼ�������д������_______���������������Ƶ���Һ��Ҫ���Ũ��Ҫ_________ (����ƫ��������ƫ����������Ӱ������������Ӧ��ȡNaOH________g��

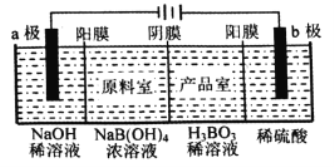
����Ŀ������ͼװ����ȡ���ᴿ���ռ����е���������(a��b��c��ʾ��Ӧ�����м�����Լ�)�����п��е���(����)

���� | a | b | c | |
A | NO2 | Ũ���� | ͭƬ | NaOH��Һ |
B | SO2 | Ũ���� | Cu | ����KMnO4��Һ |
C | NH3 | Ũ��ˮ | ��ʯ�� | ��ʯ�� |
D | O2 | ˫��ˮ | MnO2 | Ũ���� |
A. A B. B C. C D. D