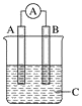
��Ŀ����
����Ŀ����1����̬Kԭ���У��������ռ������ܲ�ķ�����____��ռ�ݸ��ܲ���ӵĵ���������ͼ��״Ϊ______��
��2����̬Geԭ�ӵĺ�������Ų�ʽΪ[Ar]_______����______��δ�ɶԵ��ӡ�
��3��д����̬Asԭ�ӵĺ�������Ų�ʽ____������Ԫ�������ɣ�ԭ�Ӱ뾶Ga___As����һ������Ga____As��������ڡ���С�ڡ���
��4��Zn2+��̬��������Ų�ʽΪ____________________��
��5����ԭ�Ӽ۲���ӵĹ������ʽ�������Ų�ͼ��Ϊ_____________��
��6��CH4��CO2����������Ԫ�ص縺�Դ�С�����˳��Ϊ________________��
���𰸡�4s ���� 3d104s24p2 2 [Ar]3d104s24p3 ���� С�� [Ar]3d10 ![]() H<C<O
H<C<O
��������
��1����̬Kԭ�ӵĵ����Ų�ʽΪ[Ar]4s1������������ռ������ܲ�ķ�����4s��s���Ϊ���Σ����ռ�ݸ��ܲ���ӵĵ���������ͼ��״Ϊ���Ρ�
��2����̬Geԭ����32��Ԫ�أ�λ�ڵ������ڢ�A�壬��Cͬ�壬�����֪��������Ų�ʽΪ[Ar]3d104s24p2��p�ܼ���3����������ݺ��ع���֪��2��δ�ɶԵ��ӡ�
��3��As��ԭ������Ϊ33����̬Asԭ�ӵĺ�������Ų�ʽ[Ar]3d104s24p3��Ga��Asͬ���ڣ�ͬ���ڴ�������ԭ�Ӱ뾶��С�����ԭ�Ӱ뾶��Ga>As��ͬ���ڴ������ҵ�һ�����ܳ��������ƣ�As��4p������ڰ����״̬������ȶ�����һ�����ܣ�Ga<As��
��4��Znλ�ڵ�������IIB�壬��̬Znԭ�ӵĺ�������Ų�[Ar]3d104s2�����Zn2+��̬��������Ų�ʽΪ[Ar]3d10��
��5��Nԭ�ӻ�̬��������Ų�Ϊ1s22s22p3���۵���Ϊ2s22p3���������ʽΪ![]() ��
��
��6��CH4��CO2����������Ԫ�طֱ�ΪC��H��O������ͬ���ڣ��������ҵ縺����������縺�ԣ�C<O��Ԫ�صķǽ�����Խǿ�縺��Խ��H�ĵ縺����С����H<C<O��

 Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�����Ŀ�����Ż�����ʶ��ǿ�������ԴԽ��Խ�ܵ����ǹ�ע��
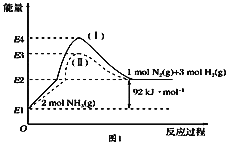
��1��������һ������Ľྻȼ�ϡ���֪��CH4��g��+2O2��g��= CO2��g��+ 2H2O��g������H= -802.3kJ��mol-1 H2O��1��=H2O��g������H =+44.0kJ��mol-l
д�����³�ѹ�¼�����ȫȼ�յ��Ȼ�ѧ����ʽ____������4.8g����������ȫȼ������Һ̬ˮ���ų�����Ϊ____kJ��
��2�����ü�����ˮ��Ӧ�Ʊ���������ԭ�����ۣ������ƹ��ֵ���÷�ӦΪCH4��g��+H2O��g��![]() CO��g��+3H2��g����H=+206.lkJ��mol-l��
CO��g��+3H2��g����H=+206.lkJ��mol-l��
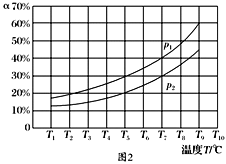
��800��ʱ����Ӧ�Ļ�ѧƽ�ⳣ��K=l.0��ijʱ�̲�ø��¶����ܱ������и����ʵ����ʵ���Ũ�����±���
CH4 | H2O | CO | H2 |
3.0 molL1 | 8.5 molL1 | 2.0 molL1 | 2.0 molL1 |
���ʱ�����淴Ӧ���ʵĹ�ϵ������Ӧ����____�淴Ӧ���ʡ��������������������������
��Ϊ��̽���¶ȡ�ѹǿ��������ѧ��Ӧ���ʵ�Ӱ�죬ijͬѧ�������������Ա�ʵ�飨�¶�Ϊ360���480�桢ѹǿΪ101 kPa��303 kPa������ʵ���������±�����
ʵ����� | �¶�/�� | ѹǿ/kPa | CH4��ʼŨ��/ molL1 | H2O��ʼŨ��/ molL1 |
1 | 360 | p | 2.00 | 6.80 |
2 | t | 101 | 2.00 | 6.80 |
3 | 360 | 101 | 2.00 | 6.80 |
����t=___��P=____�����ʵ��2��3��Ŀ����____��ʵ��l��2��3�з�Ӧ�Ļ�ѧƽ�ⳣ���Ĵ�С��ϵ��____����K1��K2��K3��ʾ����