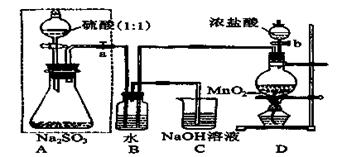
��Ŀ����
��ʵ���ҿ���ͭ��Ũ������Ȼ�������������Ʒ�Ӧ��ȡ�������������������������Ʒ�Ӧ��ȡ��������ϣ���ܿ��Ʒ�Ӧ�ٶȣ���ͼ�п�ѡ�õķ���װ���� ����д��ĸ����
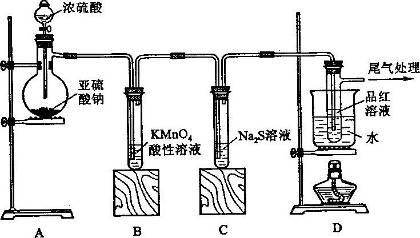
��Aͼ��ʾijѧ����SO2��Ư�۾�[80%Ca(ClO)2��]�ķ�Ӧ����ʵ��̽���Ĺ��̣��۲쵽�������У�
��.Һ���Ϸ����ְ�����
���Ժ��ֻ��ǣ���Һ��Ϊ����ɫ��
���Ժ���������ɫ����������ɫ��ȥ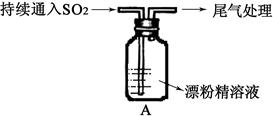
��1����ˮ�г���ͨ��SO2��δ�۲쵽�������Ʋ�������еİ�����HClСҺ���γɣ���������ʵ�飺
a����ʪ��ĵ⻯�ص�����ֽ����������ޱ仯��
b�����ữ��AgNO3��Һ���������������ɫ������
��ʵ��a��b�����жϰ����к���HCl�������� ��
��2�����д�����ɫ�����ijɷ��� ��
��3�����������Һ��Ϊ����ɫ�Ŀ���ԭ������Һ���Ե���ǿ��Ư�۾�����Ч�ɷֺ�C1-������Ӧ������Cl2��ͨ����һ��ʵ��ȷ�������ֿ����ԣ���ʵ�鷽���� ��
��4�������ӷ���ʽ����������л���ɫ��ȥ��ԭ�� ��
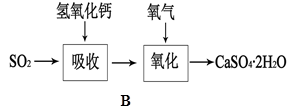
��5��Bͼ��ʾʯ��-ʯ�෨����SO2�Ĺ������̣�д����Ӧ�Ļ�ѧ����ʽ��
��
��ae (2��)
��.�� �����к���SO2Ҳ�����ữ��AgNO3��Ӧ���ɰ�ɫ����(2��)
��CaSO4 (2��)
�ǿ�ȡ����ԭ��Һ����һ������ϡ����۲쵽��Һ�����ɫ(2��)
�� Cl2+SO2+2H2O=4H++2Cl-+SO42- (2��)
�� SO2+Ca(OH)2=CaSO3��+H2O ��2CaSO3+O2+4H2O=2{CaSO4��2H2O��}(2��)
�����������������������������Ʒ�Ӧ��ȡ��������ϣ���ܿ��Ʒ�Ӧ�ٶȣ����ڷ�Ӧ����Ҫ���ȣ��ų�װ��d����������������ϸС����������ѡ��װ��bc���ʿ�ѡ�õķ���װ����ae�����������к���SO2��Ҳ�����ữ��AgNO3��Ӧ���ɰ�ɫ������������SO2��Ҳ������Ϊ���ᣬ����ʹ�����Ʒ������ֽⷴӦ��������ư�ɫ��������ȡ����ԭ��Һ����һ������ϡ���ᣬCl-��ClO-��H+�������з�Ӧ�õ�����ɫ��������ʹ��Һ�����ɫ����������Cl2�ͻ�ԭ�Ե���SO2��ˮ��Һ�з���������ԭ��Ӧ������������ᣬ�Ӷ�ʧȥ�����Ļ���ɫ��Cl2+SO2+2H2O=4H++2Cl-+SO42-��ʯ��-ʯ�෨����SO2�ĵĻ�ѧ����ʽSO2+Ca(OH)2=CaSO3��+H2O ��2CaSO3+O2+4H2O=2{CaSO4��2H2O}��
���㣺����ʵ������ȡ����Ӧ�ÿ��ǵ����ء������Ļ�ѧ���ʺ�Ӧ�ã��������SO2Σ�������Ϊ����

X��Y��Z�����ֳ����ĵ��ʣ��ס��������ֳ����Ļ�����±���������֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת������
| ѡ�� | X | Y | Z | �� | �� |
| A | H2 | Si | Cl2 | SiCl4 | HCl |
| B | Mg | C | O2 | CO2 | MgO |
| C | Zn | Fe | Cl2 | FeCl2 | ZnCl2 |
| D | Cl2 | N2 | H2 | NH3 | HCl |