��Ŀ����
����Ŀ��ij��ѧС���������ͼ1��ʾ�����ֻ�ʵ��װ�ã��о������£���1L 0.1mol/LH2A��Һ����μ����Ũ��NaOH��Һʱ��pH�仯����������Ƴ���Һ�к�AԪ�ص��������ʵ�����������ҺpH�Ĺ�ϵ��ͼ2��ʾ��������˵���в���ȷ����
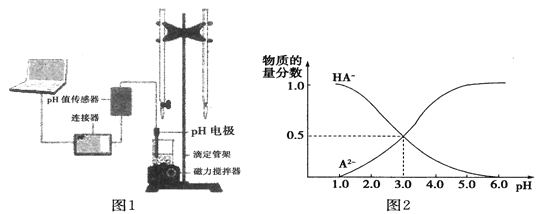
A. pH=4.0ʱ��ͼ��n(HA-)ԼΪ0.0091mol
B. ��ʵ��Ӧ����ߵ���ʽ�ζ��ܻ����ұ�ʽ�ζ��ܲ��ӷ�̪��ָʾ��
C. �����£������ʵ���Ũ�ȵ�NaHA��Na2A��Һ�������Ϻ���ҺpH=3.0
D. 0.1mol/LNaHA��Һ�д���c(A2-)+c(HA-)+c(H2A)=0.1mol/L
���𰸡�A
��������A��pH=4.0ʱ�������Һ���δ֪��������n(HA��)��ѡ��A����ȷ��B����ͼ���֪����ʵ������к��������������������Һ������Ӧ����ߵ���ʽ�ζ��ܻ����ұ�ʽ�ζ��ܲ��ӷ�̪��ָʾ����ѡ��B��ȷ��C����ͼ���֪�������£������ʵ���Ũ�ȵ�NaHA��Na2A��Һ�������Ϻ���ҺpH=3.0��ѡ��C��ȷ��D�����������غ��֪��0.1mol/LNaHA��Һ�д���c(A2��)��c(HA��)��c(H2A)��0.1mol/L��ѡ��D��ȷ����ѡA��

 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
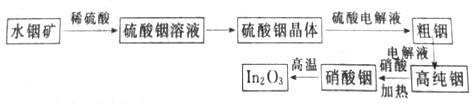
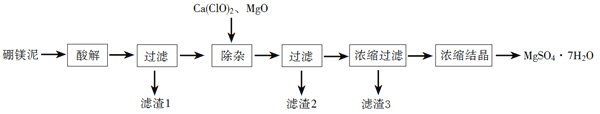
���������ν�ϵ�д�����Ŀ���Թ�ҵ������ɰ���÷�����þ��Ϊԭ����ȡMgSO4��7H2O�Ĺ�����ͼ��ʾ��
��þ�����Ҫ�ɷ����±���
MgO | SiO2 | FeO��Fe2O3 | CaO | Al2O3 | B2O3 |
30%~40% | 20%~25% | 5%~15% | 2%~3% | 1%~2% | 1%~2% |
�ش��������⣺
��1������⡱ʱӦ�ü��������_______��������1������Ҫ����_________��д��ѧʽ����
��2�������ӡ�ʱ���������ơ�����þ�����÷ֱ���________��_______��
��3���жϡ����ӡ�������ɵļ��鷽����____________��
��4����������3Ӧ���ȹ��˵�ԭ����___________��
����Ŀ����֪��ԭ���������������A��B��C��D��E���ֶ�����Ԫ�غ���Ԫ��F���������Ϣ�����ʾ��
1 | A��C�γɻ������ˮ��Һ�ʼ��� |
2 | B���������������۵Ĵ�����Ϊ0 |
3 | D�ij������������ɫ��ӦΪ��ɫ |
4 | E��ͬ�����м����Ӱ뾶��С��Ԫ�� |
(1)��������Ԫ���У���������ǿ��Ԫ�������ڱ��е�λ����__________________��
(2)����ʽΪB5Al2����һ�ȴ���ֻ��һ�ֵ��л��������Ϊ_______________________��
(3)CԪ�ص���̬�⻯����������������Ӧ��ˮ���ﻯ������M��M��ˮ��Һ��______�ԣ���M��Ũ��Һ�μӵ�Mg(OH)2����Һ�У����������Ͳ����������ԭ��__________________��
(4)DԪ�ص�����������Ӧ��ˮ������EԪ�صĵ��ʷ�Ӧ�����ӷ���ʽΪ_______________��
(5)F��B�γɵĺϽ��ڳ�ʪ�Ŀ����������绯ѧ��ʴ�γɺ���ɫ���壬��ʴ�����������ĵ缫��ӦʽΪ__________________��