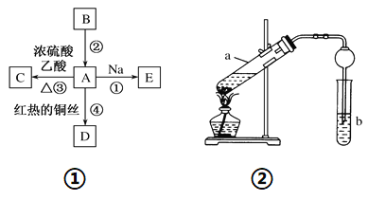
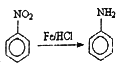
��Ŀ����
����Ŀ��(��)��֪�л��
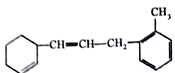
(1)�����ʱ����ϵ�һ�ȴ�����_____�֡�
(2)1mol�����ʺ���ˮ��ϣ�����Br2�����ʵ���Ϊ_____mol��
(3)1mol�����ʺ�H2�����ӳɷ�Ӧ�������H2______mol��
(4)����˵������ȷ����________(�����)��
A.�����ʿɷ����Ӿۡ��ӳɡ�ȡ���������ȷ�Ӧ
B.1mol����������2mol����
C.ʹ��ˮ��ɫ��ԭ������ϩ��ͬ
D.��ʹ����KMnO4��Һ��ɫ���������Ǽӳɷ�Ӧ
(��)ij�л���A��ѧʽΪCxHyOz��15gA��ȫȼ�տ�����22gCO2��9gH2O������
(5)���л�������ʽ________________��
(6)��A����Է�������Ϊ60�Һ�Na2CO3���������ų���A�ʹ��ܷ���������Ӧ����A�Ľṹ��ʽΪ___________��
(7)��A����Է�������Ϊ60�����ӷ���ˮ����ζ��Һ�壬�ܷ���ˮ�ⷴӦ������ṹ��ʽΪ____________��
(8)��A���ӽṹ�к���6��̼ԭ�ӣ����ж�Ԫ����ȩ�����ʣ��������������һ����Ҫ���ʡ�����ṹ��ʽΪ__________��
���𰸡� 4 2 5 BD CH2O CH3COOH HCOOCH3 CH2(OH)(CHOH)4CHO
���������������л���ṹ��ʽ��֪�����к���2��̼̼˫����1�����������ϩ���ͱ������ʷ������
(��)��5������n=m/M����CO2��H2O�����ʵ���������ԭ���غ����n��C����n��H������������m��C����m��H�������������غ�����л�������Ԫ����������������n��O�����ݴ�ȷ���л���A�����ʽ��
��6��A��Na2CO3���������ų����ʹ��ܷ���������Ӧ��˵��A�к����Ȼ��������ʵ��ʽ����Է�������ȷ��A�Ľṹ��ʽ��
��7��A���ӷ�����ˮ����ζ��Һ�壬�ܷ���ˮ�ⷴӦ��˵��A�к��������������ʵ��ʽ����Է�������ȷ��A�Ľṹ��ʽ��
��8��A���ӽṹ�к���6��̼ԭ�ӣ����ж�Ԫ����ȩ�������ʣ�˵��A�к��д��ǻ���ȩ���������ʵ��ʽȷ��A�Ľṹ��ʽ��
����1�������ʱ����ϵ�4����ԭ�Ӿ�����ͬ�������һ�ȴ�����4�֡�
��2������2��̼̼˫�������1mol�����ʺ���ˮ��ϣ�����Br2�����ʵ���Ϊ2mol��
��3��������̼̼˫���������������ӳɷ�Ӧ����1mol�����ʺ�H2�����ӳɷ�Ӧ�������H25mol��
��4��A.�����к���2��̼̼˫����1��������������ʿɷ����Ӿۡ��ӳɡ�ȡ���������ȷ�Ӧ��A��ȷ��B.1mol����������1mol������B����C.ʹ��ˮ��ɫ��ԭ������ϩ��ͬ�����Ƿ����ӳɷ�Ӧ��C��ȷ��D.��ʹ����KMnO4��Һ��ɫ����������������Ӧ��D����ѡBD��
(��)��5��n��CO2��=22g��44g/mol=0.5mol����n��C��=0.5mol��m��C��=0.5mol��12g/mol=6g��n��H2O��=9g��18g/mol=0.5mol����n��H��=1mol��m��H��=1mol��1g/mol=1g�����������غ��֪��A��m��O��=15g-6g-1g=8g����n��O��=8g��16g/mol=0.5mol����n��C����n��H����n��O��=0.5mol��1mol��0.5mol=1��2��1�����A�����ʽΪCH2O��
��6��A�����ʽ��ʽ��Ϊ30����A����Է�������Ϊ60����A�ķ���ʽΪC2H4O2����A��Na2CO3���������ų����ʹ��ܷ���������Ӧ��˵��A�к���-COOH����AΪ���ᣬ��ṹ��ʽΪCH3COOH��
��7�����ݣ�2����֪A�ķ���ʽΪC2H4O2����A���ӷ�����ˮ����ζ��Һ�壬�ܷ���ˮ�ⷴӦ��˵��A�к�������������������AΪ�����������ṹ��ʽΪHCOOCH3��
��8����A���ӽṹ�к���6��̼ԭ�ӣ����ж�Ԫ����ȩ�������ʣ�˵��A�к��д��ǻ���ȩ������A��ʵ��ʽΪCH2O����AΪ�����ǣ���ṹ��ʽΪCH2(OH)(CHOH)4CHO��