��Ŀ����
15����ϸ��������ĩ���㷺Ӧ���ڴ��ģ���ɵ�·��������������ȡԭ��Ϊ��Al2O3+N2+3C$\frac{\underline{\;����\;}}{\;}$2AlN+3CO�����ڷ�Ӧ����ȫ����������Ʒ�������������ʣ���ȡ��ʵ���Ʒ������������ʵ�飺
��1�����ò�Ʒ�ڹ�����NaOHŨ��Һ��Ӧ�����ⶨ�а������ɣ�д����ʵ�������������п��ܵĻ�ѧ����ʽAlN+NaOH+H2O�TNaAlO2+NH3����Al2O3 +2NaOH�T2 NaAlO2 +H2O��
��2��ȡ�ò�Ʒ12.00g���ڷ�Ӧ���У�ͨ���״����6.048L O2�����������ڸ����³�ַ�Ӧ����������ܶ�Ϊ1.50g•L-1��������ɱ�״����AlN����O2��Ӧ�������ʵ���ȷ�ϸò�Ʒ�к��е�������C��̿�������ʵ���������Ϊ3.6%���������С�����һλ����
���� ��1�����ڷ�Ӧ����ȫ����������Ʒ����������������������������������������Һ��Ӧ����ƫ�����ƺͰ�����������������������Һ��Ӧ����ƫ�����ƺ�ˮ��
��2���������������ܶȿɼ������������ƽ����Է�������Ϊ33.6����������������������ض���O2��CO2������壬�����������ʵ����õ�������������ȥ��Ԫ�������õ�̼���������ݴ˼�����Ʒ��̼��������
��� �⣺��1�����ڷ�Ӧ����ȫ����������Ʒ����������������������������������������Һ��Ӧ����ƫ�����ƺͰ�������Ӧ�Ļ�ѧ����ʽΪ��AlN+NaOH+H2O�TNaAlO2+NH3����������������������Һ��Ӧ����ƫ�����ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��Al2O3 +2NaOH�T2NaAlO2 +H2O��
�ʴ�Ϊ��AlN+NaOH+H2O�TNaAlO2+NH3����Al2O3 +2NaOH�T2NaAlO2 +H2O��
��2���������������ܶȿɼ������������ƽ����Է�������=1.50g•L-1��22.4L/mol=33.6����������������������ض���O2��CO2������壬ȷ�ϸò�Ʒ�к��е�������C��
O2���ʵ���=$\frac{6.048L}{22.4L/mol}$=0.27mol
���ԣ���������=0.27mol��33.6g/mol=9.072g����Ʒ��C������Ϊ9.072g-0.27mol��2��16g/mol=0.432g��
�����ʵ���������=$\frac{0.432g}{12g/mol}$��100%=3.6%��
�ʴ�Ϊ��C��̿��3.6%��
���� ����Ϊ���͵���Ʒ���Ȳ������⣬����ʵ��ԭ���ǹؼ����Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������ѧϰ��ȫ����ջ���֪ʶ���Ѷ��еȣ�

| A�� | һ���¶��£�1L0.5mol•L-1NH4Cl��Һ��1L0.25mol•L-1NH4Cl��Һ��NH4+���ʵ���֮��Ϊ2��1 | |
| B�� | 100g������Һ�����ʵ���Ũ��Ϊ18.4mol/L����ˮϡ�͵����ʵ���Ũ��Ϊ9.8mol/L����Ҫˮ100g�� | |
| C�� | ��״���£�22.4LCH3Cl��CHCl3�Ļ�����������е�̼ԭ�Ӹ���һ��ΪNA | |
| D�� | ��2Na2O2+2CO2=2Na2CO3+O2��Ӧ�У�ÿ����16g��������ת��NA������ |
| A�� | ú��������Һ�������������仯 | |
| B�� | ͨ��ʯ�ͷ���õ��������Ǵ����� | |
| C�� | ʯ���ѽ���Ϊ�˵õ���ϩ����ϩ����̬������ | |
| D�� | ����IJ�������ȡ�������ױ��ȷ���������Ϊú�к��б��ͼױ� |
| A�� | NaClO���ڹ��ۻ����� | |
| B�� | ��84������Һ�ڿ����о��û���� | |
| C�� | 1L 0.1mol?L-1NaClO��Һ��ClO-����ĿΪ0.1NA | |
| D�� | ClO-��Ca2+��Fe2+��Al3+ �ܴ������� |
| A�� | �ڹ���Ԫ����Ѱ�Ұ뵼����� | |
| B�� | Ԫ�����ڱ��е�һ��Ԫ�ض��Ǽ����Ԫ�� | |
| C�� | ����Ԫ�����ڱ��ǰ���Ԫ�����ԭ��������С�����˳������ | |
| D�� | M�����Ϊ��������������Ԫ�ص����������Ԫ��ԭ�ӵ�M���������� |
| A�� | ��״���£�22.4LSO3����NA������ | |
| B�� | ��״���£�22.4LCO2��CO�Ļ�����庬NA������ | |
| C�� | lmolNa2O2������CO2��Ӧת�Ƶĵ�����Ϊ2NA | |
| D�� | ������NA��H2���ӵ�����ԼΪ2g������ռ�����ԼΪ22.4L |
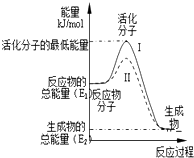 ��1����֪��
��1����֪��