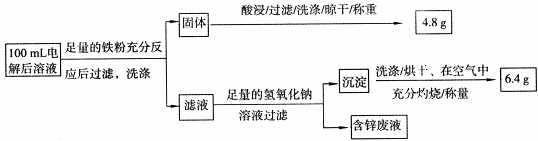
��Ŀ����
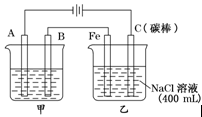
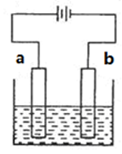
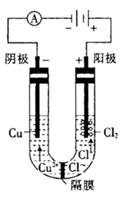
����ͼװ���У�b�缫�ý��� M�Ƴɣ�a��c��dΪʯī�缫����ͨ��Դ������M������b����ͬʱa��d�缫�ϲ������ݡ��Իش�
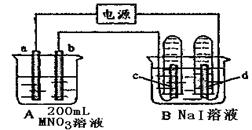
��1��aΪ ����c���ĵ缫��ӦʽΪ ��
��2��������һ��ʱ�������c���ϵ��Թ���Ҳ���ռ��������壬��ʱc���ϵĵ缫��ӦʽΪ ��
��3����d�����ռ���44.8mL���壨��״����ʱֹͣ��⣬a���Ϸų�����������ʵ���Ϊ ����b�缫�ϳ�������M������Ϊ0.432g����˽�����Ħ������Ϊ ��

��1��aΪ ����c���ĵ缫��ӦʽΪ ��
��2��������һ��ʱ�������c���ϵ��Թ���Ҳ���ռ��������壬��ʱc���ϵĵ缫��ӦʽΪ ��
��3����d�����ռ���44.8mL���壨��״����ʱֹͣ��⣬a���Ϸų�����������ʵ���Ϊ ����b�缫�ϳ�������M������Ϊ0.432g����˽�����Ħ������Ϊ ��
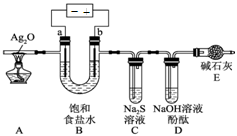
��1������1�֣��� 2I��һ2e����I2��2�֣�
��2��40H����4e����2H2O+O2�� ��2�֣�
��3��0��001 mol��1�֣���108g��mol ��2�֣�
��2��40H����4e����2H2O+O2�� ��2�֣�
��3��0��001 mol��1�֣���108g��mol ��2�֣�
����������ɵ��ԭ���ɵã�����M������b����˵��b����������a��������c��������d����������1����a����������Һ�е������ӷŵ磬�������ӵķŵ�˳��֪��2I--2e-=I2����2����B�ձ��У� c����������Һ�е������ӷŵ磬��2I--2e-=I2��I2����������ʹ���۱�����I-�ŵ���Ϻ�����OH-�ŵ磺4OH--4e=2H2O+O2����c���ϵ��Թ����ռ���������Ϊ��������3��d�缫���ռ���44.8ml���壨��״������������a�����ռ���������������������ת�Ƶ��������֪�����������������֮����1��2��d�缫���ռ���44.8ml������������a�缫���ռ���22.4mL���������ʵ���Ϊ0.01mol��d�缫�����������������ʵ���=
 ��ת�Ƶ��ӵ����ʵ�����0.04mol����������M��+1�ۣ����Ե�ת��0.04mol����ʱ����0.04mol�������ʣ�M=
��ת�Ƶ��ӵ����ʵ�����0.04mol����������M��+1�ۣ����Ե�ת��0.04mol����ʱ����0.04mol�������ʣ�M=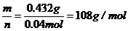

��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
�����Ŀ

 ������ȫ������pH��3.2ʱ
������ȫ������pH��3.2ʱ
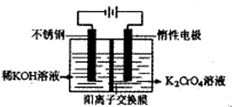
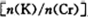 Ϊd�����ʱ�ĸ���ص�ת����Ϊ ��
Ϊd�����ʱ�ĸ���ص�ת����Ϊ ��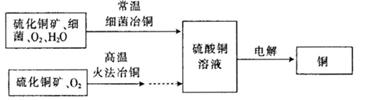
 6Cu+SO2������Ӧ���������� ��
6Cu+SO2������Ӧ���������� ��