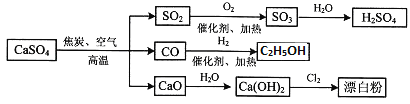
��Ŀ����
����Ŀ��ͪ�����һ�����õĿ�����ʹҩ������ͨ�����·����ϳɣ�

��1��ͪ����к��������ŵ�����Ϊ______________��______________��
��2����B��C�ķ�Ӧ������____________________��
��3��д��D��E��Ӧ�Ļ�ѧ����ʽ______________________________________________��
��4��д��ͬʱ��������������A��һ��ͬ���칹��Ľṹ��ʽ_____________________��
��.�ܷ���������Ӧ
��.ˮ�����֮һ��FeCl3��Һ��ɫ
��.�����к���4�ֲ�ͬ��ѧ��������ԭ��
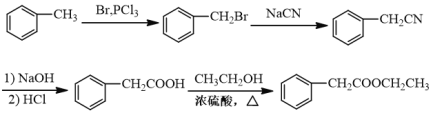
��5����д���Լױ����Ҵ�Ϊԭ���Ʊ�������![]() �ĺϳ�·������ͼ�����Լ�����ѡ��________________��
�ĺϳ�·������ͼ�����Լ�����ѡ��________________��
���𰸡��Ȼ� �ʻ� ȡ����Ӧ ![]()
![]()
��������
��1������ͪ��ҵĽṹ��ʽ����֪���еĺ���������Ϊ�Ȼ����ʻ���
��2���Ա�B��C�ṹ��ʽ��֪��B��-ClΪ����ȡ������C��ͬʱ������HCl��
��3���Ա�D��F�ṹ��E�ķ���ʽ����֪D��-Br��-CNȡ������E��
��4��A��һ��ͬ���칹�����㣺���ܷ���������Ӧ��˵������ȩ������ˮ�����֮һ��FeCl3��Һ��ɫ��˵�����м�������ṹ�������к���4�ֲ�ͬ��ѧ�������⣬������-OOCH��-CH3���ڶ�λ��
��5������ɿ�֪��ʱ������Ŀ����ﻹ�豽���ᣬ�ɼױ����ɱ�������Ҫ�ڼ���������һ��̼ԭ�ӣ����ͪ��ҵĺϳ�·��B��C��D��E��F������ɼױ��ϳɱ����ᣬ��ͨ��������Ӧ�������Ŀ�������
��1������ͪ��ҵĽṹ��ʽ����֪���еĺ���������Ϊ�Ȼ����ʻ���
�ʴ�Ϊ���Ȼ����ʻ���
��2���Ա�B��C�ṹ��ʽ��֪��B��-ClΪ����ȡ������C��ͬʱ������HCl����Ӧ������ȡ����Ӧ��
�ʴ�Ϊ��ȡ����Ӧ��
��3���Ա�D��F�ṹ��E�ķ���ʽ����֪D��-Br��-CNȡ������E����Ӧ�Ļ�ѧ����ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
��4��A��һ��ͬ���칹�����㣺���ܷ���������Ӧ��˵������ȩ������ˮ�����֮һ��FeCl3��Һ��ɫ��˵�����м�������ṹ�������к���4�ֲ�ͬ��ѧ�������⣬������-OOCH��-CH3���ڶ�λ�����������Ľṹ��ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
��5���ױ���Br2/PCl3����ȡ����Ӧ����![]() ����NaCN����ȡ����Ӧ����
����NaCN����ȡ����Ӧ����![]() ���ٸ��ݢ�NaOH/��HCl�õ�
���ٸ��ݢ�NaOH/��HCl�õ�![]() ��������Ҵ�����������Ӧ�õ�
��������Ҵ�����������Ӧ�õ�![]() ��
��
�ϳ�·������ͼΪ��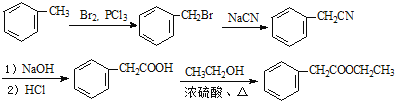 ��
��
�ʴ�Ϊ�� ��
��

 ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�����Ŀ������(�ֳ��£�N2H4����ɫҺ��)��һ��Ӧ�ù㷺�Ļ���ԭ�ϣ����������ȼ��������ƽ�����װ�л�ԭ����(N2H4)��ǿ��������������(H2O2)�������ǻ��ʱ������������������ˮ���������ų������ȡ���֪0.5 molҺ̬���������������ⷴӦ�����ɵ�����ˮ�������ų�320.8 kJ��������
��1���µĵ���ʽΪ___________________����������ĵ���ʽΪ__________________��
��2��д����Ӧ���Ȼ�ѧ����ʽ��________________________________________________��
��3����25 �桢101 kPaʱ����֪18 gˮ�������Һ̬ˮ�ų�44 kJ����������������������±���
O===O | H��H | H��O(g) | |
1 mol��ѧ������ʱ ��Ҫ���յ�����/kJ | 496 | 436 | 463 |
д����ʾH2ȼ���ȵ��Ȼ�ѧ����ʽ___________________________________________����32 gҺ̬��������Һ̬�������ⷴӦ���ɵ�����Һ̬ˮʱ���ų���������________kJ��
��4��������H2O2����Ϊ����ƽ�������Ҫԭ��Ϊ_________________________________��
��5������Ϊ��Ԫ�����ˮ�еĵ��뷽��ʽ�백���ƣ�������һ�����뷴Ӧ��ƽ�ⳣ��ֵΪ____________(��֪��N2H4+H+![]() N2H5+��K=8.7��107��KW=1.0��10-14)�������������γɵ���ʽ�εĻ�ѧʽΪ_______________��
N2H5+��K=8.7��107��KW=1.0��10-14)�������������γɵ���ʽ�εĻ�ѧʽΪ_______________��
����Ŀ�������������������Ʊ������롢�ᴿ��������֤��ʵ��װ�ã������й�˵����ȷ����

ѡ�� | ���� | װ�� | ʵ��IJ��������� |
A | �Ʊ� | a | �Թ���NaHCO3��Һ�����ã���Ӧ���ӷ������ᣬ�ܽ��Ҵ��������������������ռ� |
B | ���� | b | Ӧ���¶ȼƲ���Һ�����£��Ա�ȷ�������ֵ��¶� |
C | ��Һ | c | ��Һ©�����������������������������ձ��� |
D | ˮ�� | d | ����������ˮ����ȫ�ı�־Ϊ��Һ���ٷֲ� |
A.AB.BC.CD.D
����Ŀ��CO2�����������ǻ�����ѧ���о����ȵ���⡣����CO2�Ʊ��ϳ�����CO��H2�������Ʊ���ֵ��Ʒ����״��ȣ�Ҳ��������CO2ֱ���Ʊ��״��Ȳ�Ʒ��
��1����֪���ַ�Ӧ������Ӧ��ܣ�E1�����淴Ӧ��ܣ�E2�������ʾ��
��� | ��ѧ��Ӧ | E1/��kJ��mol-1�� | E2/��kJ��mol-1�� |
�� | 2CO��g��+O2��g��===2CO2��g�� | 1954 | 2519 |
�� | H2(g)+ | 685 | 970 |
�� | 2CH3OH��g��+3O2��g��===2CO2��g��+4H2O��l�� | 3526 | 4978 |
����ͬ�����£���ʼ��Ӧ������____������ţ�����H2��CO�ϳ���̬�״����Ȼ�ѧ����ʽΪ________��
��2��T1���£���2L�����ܱ������г���0.20mol CO��0.60mol H2�ϳ�CH3OH��������Ӧ��CO��g��+2H2��g��![]() CH3OH��g�� ��H<0����5minǡ�ôﵽƽ�⣬CH3OH��Ũ����0.05mol��L-1��
CH3OH��g�� ��H<0����5minǡ�ôﵽƽ�⣬CH3OH��Ũ����0.05mol��L-1��
��T1��ʱ����H2��ʾ�ķ�Ӧ��ƽ������Ϊ___��ƽ�ⳣ��K=____��
�ڣ�T1+100����ʱ����1L�����ܱ������г���0.10molCO��0.20molH2��0.30mol CH3OH����ʱ��Ӧ��____��������ƶ����������ƶ������ﵽƽ�⡱�����жϡ�����
��3��CO2��H2�ڴ��������·�Ӧ������ֱ�Ӻϳɼ״���CO��g��+3H2��g��![]() CH3OH��g��+H2O��g�� ��H<0�������ͬʱ���ڼ״��������¶ȵĹ�ϵ��ͼ��ʾ��
CH3OH��g��+H2O��g�� ��H<0�������ͬʱ���ڼ״��������¶ȵĹ�ϵ��ͼ��ʾ��
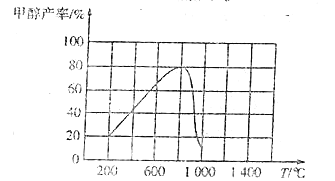
���¶���800��ʱ�״�������ߵ���Ҫԭ����/span>____��
�����д�ʩ�����CO2ƽ��ת���ʵ���____������ĸ����
A������ B����ѹ C��������� D������H2Ũ��
��4�����õ�ⷨ�����������½�CO2��H2Oת����CO��H2��������Ӧʽ֮һΪCO2+2e-+2H+=CO+H2O����������£���CO2+2e-+H2O===CO+2OH-������Ӧ������OH-+CO2=HCO3-���췴Ӧ������HCO3-+H+=CO2+H2O���췴Ӧ��������ˮ��������___���������巴Ӧ���ʵķ�Ӧ��___������ţ���
����Ŀ��������ͼ��ʾװ�ý�������ʵ�飬�ܵó���Ӧʵ����۵���( )
ѡ�� | �� | �� | �� | ʵ����� | ʵ��װ�� |
A | ϡ���� | Na2S | AgNO3��AgCl����Һ | Ksp(AgCl)��Ksp(Ag2S) |
|
B | Ũ���� | ���� | ��ˮ | Ũ���������ˮ�ԡ������� | |
C | ϡ���� | Na2SO3 | Ba(NO3)2 ��Һ | SO2������Ա��ξ��������ɰ�ɫ���� | |
D | Ũ���� | Na2CO3 | Na2SiO3��Һ | ���ԣ����̼����� |
A. A B. B C. C D. D