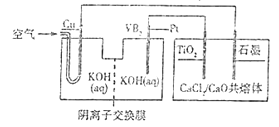
��Ŀ����
����Ŀ������(�ֳ��£�N2H4����ɫҺ��)��һ��Ӧ�ù㷺�Ļ���ԭ�ϣ����������ȼ��������ƽ�����װ�л�ԭ����(N2H4)��ǿ��������������(H2O2)�������ǻ��ʱ������������������ˮ���������ų������ȡ���֪0.5 molҺ̬���������������ⷴӦ�����ɵ�����ˮ�������ų�320.8 kJ��������
��1���µĵ���ʽΪ___________________����������ĵ���ʽΪ__________________��
��2��д����Ӧ���Ȼ�ѧ����ʽ��________________________________________________��
��3����25 �桢101 kPaʱ����֪18 gˮ�������Һ̬ˮ�ų�44 kJ����������������������±���
O===O | H��H | H��O(g) | |
1 mol��ѧ������ʱ ��Ҫ���յ�����/kJ | 496 | 436 | 463 |
д����ʾH2ȼ���ȵ��Ȼ�ѧ����ʽ___________________________________________����32 gҺ̬��������Һ̬�������ⷴӦ���ɵ�����Һ̬ˮʱ���ų���������________kJ��
��4��������H2O2����Ϊ����ƽ�������Ҫԭ��Ϊ_________________________________��
��5������Ϊ��Ԫ�����ˮ�еĵ��뷽��ʽ�백���ƣ�������һ�����뷴Ӧ��ƽ�ⳣ��ֵΪ____________(��֪��N2H4+H+![]() N2H5+��K=8.7��107��KW=1.0��10-14)�������������γɵ���ʽ�εĻ�ѧʽΪ_______________��
N2H5+��K=8.7��107��KW=1.0��10-14)�������������γɵ���ʽ�εĻ�ѧʽΪ_______________��
���𰸡�![]()
![]() N2H4(l)��2H2O2(l)===N2(g)��4H2O(g) ��H����641.6 kJ��mol��1 H2 (g) +
N2H4(l)��2H2O2(l)===N2(g)��4H2O(g) ��H����641.6 kJ��mol��1 H2 (g) + ![]() O2 (g) = H2O (l) ��H= -286 kJmol-1 817.6 ��Ӧ�����������������壨������N2��H2O���Ի�������Ⱦ�� 8.7��10-7 N2H6(HSO4)2
O2 (g) = H2O (l) ��H= -286 kJmol-1 817.6 ��Ӧ�����������������壨������N2��H2O���Ի�������Ⱦ�� 8.7��10-7 N2H6(HSO4)2
��������
(1)�·���ʽΪN2H4��ÿ����ԭ���γ�������ѧ���������������ʽH2O2��ÿ����ԭ���γ��������ۼ����ݴ�д�����ʵĵ���ʽ��
(2)�����Ȼ�ѧ����ʽ��д����д������ע���ʾۼ�״̬�ͷ�Ӧ�ʱ���
(3) ��Ӧ����ܼ���-��������ܼ���=��Ӧ�����ݴ˼��㷴Ӧ����д���Ȼ�ѧ����ʽ�������Ȼ�ѧ����ʽ��˹���ɼ���õ��Ȼ�ѧ����ʽ���õ���Ӧ���ʱ���
(4)���ṩ�����Ͷ����Լ���������Ⱦ�Ƕȷ�����
(5) ����Ϊ��Ԫ��������ˮ�еĵ��뷽ʽ�백������������һ�����뷽��ʽΪN2H4+ H2O ![]() N2H5++OH-��ƽ�ⳣ��Kb=
N2H5++OH-��ƽ�ⳣ��Kb=![]() =
=![]() ��
��![]() =K��Kw��
=K��Kw��
��Ϊ�Ƕ�Ԫ������������������γɵ���ʽ��ΪN2H6(HSO4)2��
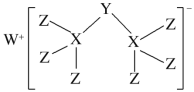
(1)���ǵ�ԭ�Ӻ͵�ԭ���γ�һ�����ۼ���ʣ��ۼ�����ԭ���γɹ��ۼ�������ʽΪ![]() ��˫��ˮΪ���ۻ������������д��������������һ��O-O����˫��ˮ�ĵ���ʽΪ��
��˫��ˮΪ���ۻ������������д��������������һ��O-O����˫��ˮ�ĵ���ʽΪ��![]() ��
��
��ˣ�������ȷ������![]() ��
��![]() ��
��
(2) 0.5 molҺ̬���������������ⷴӦ�����ɵ�����ˮ�������ų�320.8 kJ��������1mol��ȼ�շ���Ϊ641.6 kJ����ȼ�յ��Ȼ�ѧ����ʽΪ��N2H4(l)��2H2O2(l)===N2(g)��4H2O(g) ��H����641.6 kJ��mol��1��
��ˣ�������ȷ������N2H4(l)��2H2O2(l)===N2(g)��4H2O(g) ��H����641.6 kJ��mol��1��
(3) ���ݷ�Ӧ����ܼ���-��������ܼ���=��Ӧ�ȣ���1mol����ȼ������1molˮ�����ķ�Ӧ��Ϊ436kJ/mol+496kJ/mol��![]() -463kJ/mol��2=-242kJ/mol��18gˮ�������Һ̬ˮ�ų�44kJ����������1mol����ȼ������1molҺ̬ˮʱ�ų�����Ϊ242kJ+44kJ=286kJ��
-463kJ/mol��2=-242kJ/mol��18gˮ�������Һ̬ˮ�ų�44kJ����������1mol����ȼ������1molҺ̬ˮʱ�ų�����Ϊ242kJ+44kJ=286kJ��
�ʱ�ʾH2ȼ���ȵ��Ȼ�ѧ����ʽΪH2 (g) + ![]() O2 (g) = H2O (l) ��span>H= -286 kJmol-1��
O2 (g) = H2O (l) ��span>H= -286 kJmol-1��
��֪��N2H4(l)��2H2O2(l)===N2(g)��4H2O(g) ��H����641.6 kJ��mol��1��
��H2O(l)�TH2O(g) ��H��+44kJ��mol-1�����ݸ�˹��������-�ڡ�4�õ���N2H4(l)��2H2O2(l)===N2(g)��4H2O(l) ��H����817.6 kJ��mol��1��
��32gҺ̬��������Һ̬�������ⷴӦ���ɵ�����Һ̬ˮ����Ϊ817.6 kJ��
��ˣ�������ȷ������H2 (g) + ![]() O2 (g) = H2O (l) ��H= -286 kJmol-1 ��817.6��
O2 (g) = H2O (l) ��H= -286 kJmol-1 ��817.6��
��4��N2H4����ǿ��ԭ�Ժ�H2O2����������ԭ��Ӧ�ų��������Ҳ����������壬��˿���Ϊ����ƽ�����
��ˣ�������ȷ��������Ӧ�����������������壨������N2��H2O���Ի�������Ⱦ����
��5������Ϊ��Ԫ��������ˮ�еĵ��뷽ʽ�백������������һ�����뷽��ʽΪN2H4+ H2O ![]() N2H5++OH-��ƽ�ⳣ��Kb=
N2H5++OH-��ƽ�ⳣ��Kb=![]() =
=![]() ��
��![]() =K��Kw=8.7��107��1.0��10-14=8.7��10-7��
=K��Kw=8.7��107��1.0��10-14=8.7��10-7��
��Ϊ�Ƕ�Ԫ������������������γɵ���ʽ��ΪN2H6(HSO4)2��
��ˣ�������ȷ������8.7��10-7 ��N2H6(HSO4)2��