��Ŀ����
����Ŀ��ͭ�����ֳ�����������CuO��Cu2O��ijѧϰС��ȡ0.98 g(�þ�����ƽ����)Cu(OH)2������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ1��ʾ�����⣬ijͬѧ������������ʾ����������������������Ԫ�������Ĺ�ϵ���ߣ���ͼ2��ʾ�������з�����ȷ����(����)
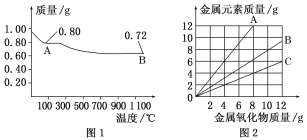
A. ͼ1�У�A��B�Ĺ�������0.005 mol���ӷ�����ת��
B. ͼ1���������й�����0.18 gˮ
C. ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������A
D. ͼ1��A��B��ѧʽ�ֱ�ΪCu2O��CuO
���𰸡�B
��������
0.98 g Cu(OH)2���ʵ���Ϊ0.98g��98g/mol=0.01 mol����ȫ������CuO��������Ϊ0.01 mol��80 gmol-1=0.8g������A����CuO����ȫ������Cu2O��������Ϊ0.005 mol��144 gmol-1=0.72g������B����Cu2O��
A.���ݷ�Ӧ����ʽ4CuO![]() 2Cu2O+O2����֪��A��B�Ĺ�����ת�Ƶ������ʵ���Ϊ0.01 mol��A����
2Cu2O+O2����֪��A��B�Ĺ�����ת�Ƶ������ʵ���Ϊ0.01 mol��A����
B.���ݻ�ѧ����ʽCu(OH)2![]() CuO+H2O��4CuO
CuO+H2O��4CuO![]() 2Cu2O+O2����֪��ˮ�����ʵ���Ϊ0.01mol������Ϊ0.01mol��18g/mol=0.18g��B��ȷ��
2Cu2O+O2����֪��ˮ�����ʵ���Ϊ0.01mol������Ϊ0.01mol��18g/mol=0.18g��B��ȷ��
C.10gCuO������CuԪ�ص�����Ϊ��![]() ��64g=8g���۲�ͼ2��֪����ʾCuO����B���ߣ�C����
��64g=8g���۲�ͼ2��֪����ʾCuO����B���ߣ�C����
D.��������������A��B�Ļ�ѧʽ����ΪCuO��Cu2O��D����
��ѡB��

 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�����Ŀ���й���ͳ�Ļ��������������Ŵ������м����˹Ŵ���ѧ�о��ɹ������г�����ʫ�Ķ�Ӧ�Ļ�ѧ֪ʶ��ȷ����
������ʫ�ļ��� | ��ѧ֪ʶ | |
A | ����Ϫ��̸���жԱ����ļ��أ��������Լ���Ϊ�У�����Ϊ���ɣ����������ۡ� | ���ĺϽ�Ӳ�ȱȴ����Ĵ��۵�ȴ����ĸ� |
B | �����ݸ�Ŀʰ�š��ж�ǿˮ�ļ��أ��������ң���ʴ�����ˮ��ǿ��Ω������ʢ�� | ǿˮΪ����� |
C | ���칤����м��أ�����ҩ����Ϊ��������Ϊ���� | ��ָ������ƣ���ָ��������� |
D | ��Ȫ�ݸ�־���м��أ������˻�����Ϊլ���ǣ�ǽ��ѹ�ǣ�ȥ�����ǰף�������Ч֮ | �ǰĹ��̷����˻�ѧ�仯 |
A. AB. BC. CD. D
����Ŀ�����ײ���һֱ�������о�����Ҫ���⣬��������Fe�۱�������г�ǿ�Ĵ��ԣ���Ч���Ե����������ʡ�
I��ʵ���Ҳ������ԭ���Ʊ�����Fe����������ͼ��ʾ��
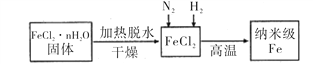
��1������Fe��ϡ���ᷴӦ�����ӷ���ʽΪ_______________________________��
��2����ν�FeCl2��nH2O���������ˮ�Ƶ���ˮFeCl2 _____________________________________(�ü�Ҫ��������)��
��3����������Fe�Ļ�ѧ����ʽΪ______________________________________��
II���������ϣ��ڲ�ͬ�¶��£�����Fe����ˮ������Ӧ�Ĺ�����ﲻͬ���¶ȵ���570��ʱ����FeO������570��ʱ����Fe3O4����ͬѧ����ͼ��װ����ʾ��������Fe����ˮ������Ӧ��ʵ�飬��ͬѧ��ͼ����ʾ��װ�ý�������Fe����ˮ�����ķ�Ӧ����֤���
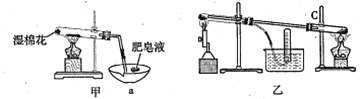
��4����װ��������Fe����ˮ������Ӧ�Ļ�ѧ����ʽ�� ______________________��
��5����װ��������a������Ϊ_______________________��
��6����ͬѧΪ̽��ʵ��������Թ��ڵĹ������ʳɷ֣�����������ʵ�飺
ʵ�鲽�� | ʵ����� | ʵ������ |
I | ����Ӧ��õ��ĺ�ɫ��ĩX(�ٶ�Ϊ���ȵ�)��ȡ������������һ�Թ��У������������ᣬ�� | ��ɫ��ĩ���ܽ⣬��Һ��dz��ɫ�����������ݲ��� |
II | ��ʵ��I�õ�����Һ�еμӼ���KSCN��Һ���� | ��Һû�г��ֺ�ɫ |
��������ʵ�飬��ͬѧ��Ϊ�������·�Ӧ�Ĺ������ΪFeO��
��ͬѧ��Ϊ��ͬѧ�Ľ��۲���ȷ������������______(�ü�Ҫ��������)��
��7����ͬѧ��ȡ5.60gFe�ۣ�����װ��Ӧһ��ʱ���ֹͣ���ȡ����Թ��ڵĹ��������ڸ���������ȴ�Ƶ�����Ϊ6.88g����ͬѧʵ���Ĺ������������������������Ϊ________(���������λ��Ч����)��