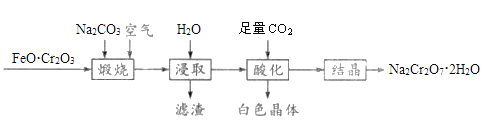
��Ŀ����
����Ŀ��������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50mL0.25mol��L-1���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50mL0.55mol��L-1NaOH��Һ��������һ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ������¶ȡ�
�ش��������⣺
��1������NaOH��Һ����ȷ������___��
A.�ز������������� B.�������������� C.һ��Ѹ�ٵ���
��2��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������__��
A.���¶ȼ�С�Ľ��� B.�ҿ�ӲֽƬ�ò���������
C.��������ձ� D.�������¶ȼ��ϵĻ��β������������������ƶ�
��3��ʵ���������±���
������д�±��еĿհף�
�¶� ʵ����� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ(t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | ___________ |
2 | 25.9 | 25.9 | 25.9 | 29.2 | |
3 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ڽ��Ƶ���Ϊ0.55mol��L-1NaOH��Һ��0.25mol��L-1������Һ���ܶȶ���1g��cm-3���кͺ�������Һ�ı�����c=4.18J��g-1����-1�����к�����H=__(ȡС�����һλ)��
���𰸡�C D 3.4 -56.8kJ/mol
��������
(1)��������������Һʱ������һ��Ѹ�ٵĵ��룬Ŀ���Ǽ���������ɢʧ��
(2)�����к��Ȳⶨ��ȷ��������������
(3)��3���¶Ȳ�ֱ�Ϊ��3.4����3.3����3.5��������Ч�������¶Ȳ�ƽ��ֵ��
�ڸ��ݹ�ʽ���м��㡣
(1)��������������Һʱ������һ��Ѹ�ٵĵ��룬Ŀ���Ǽ���������ɢʧ�����ּܷ��ε�������������Һ������ᵼ������ɢʧ��Ӱ��ⶨ������ʴ�Ϊ��C��
(2)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ���������ǣ��������¶ȼ��ϵĻ��β������������ؽ������¶ȼ��Dz����¶ȵģ�����ʹ���¶ȼƽ��裻Ҳ������������ձ���������ܵ���Һ�彦��������ɢʧ��Ӱ��ⶨ����������ܴ�ӲֽƬ�ò��������裬�����������ɢʧ���ʴ�Ϊ��D��
(3)��3���¶Ȳ�ֱ�Ϊ��3.4����3.3����3.5��������Ч���¶Ȳ�ƽ��ֵ=3.4�����ʴ�Ϊ��3.4��
��50mL0.25mol/L������50mL0.55mol/L NaOH��Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.25mol/L��2=0.025mol����Һ������Ϊ��100ml��1g/ml=100g���¶ȱ仯��ֵΪ��T=3.4����������0.025molˮ�ų�������ΪQ=mc��T=100g��4.18J/(g��)��3.4��=1421.2J����1.4212kJ������ʵ���õ��к��ȡ�H=-1.4212kJ/0.025mol=-56.8kJ/mol���ʴ�Ϊ��-56.8kJ/mol��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�